Tablets containing clopidogrel hydrogen sulfate and preparation method thereof
A technology of clopidogrel bisulfate and tablets, which is applied in the direction of medical preparations containing active ingredients, medical preparations without active ingredients, pill delivery, etc. Problems such as sticking and rushing occur, so as to improve drug sticking and rushing, reduce surface area, and prevent deliquescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a kind of preparation method of clopidogrel hydrogen sulfate tablet, comprises the following steps:
[0024] a) Polyethylene glycol is mixed with a solvent to obtain a polyethylene glycol solution.
[0025] b) Clopidogrel bisulfate or other pharmaceutical excipients are placed in an ebullating bed, the polyethylene glycol solution is added, and dried to obtain non-sticky clopidogrel bisulfate granules.
[0026] c) The non-sticky clopidogrel hydrogen sulfate granules are mixed with other pharmaceutical excipients, and the mixed product is compressed into tablets to obtain clopidogrel hydrogen sulfate tablets.
[0027] In the preparation method of the non-sticky clopidogrel bisulfate granules provided by the present invention, polyethylene glycol is first mixed with a solvent to obtain a polyethylene glycol solution. Wherein, the number average molecular weight of the polyethylene glycol is preferably 1500-8000, more preferably 4000-6000; in the pr...
Embodiment 1
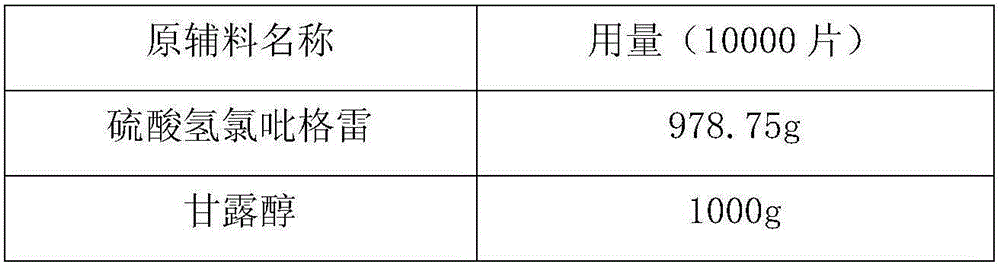
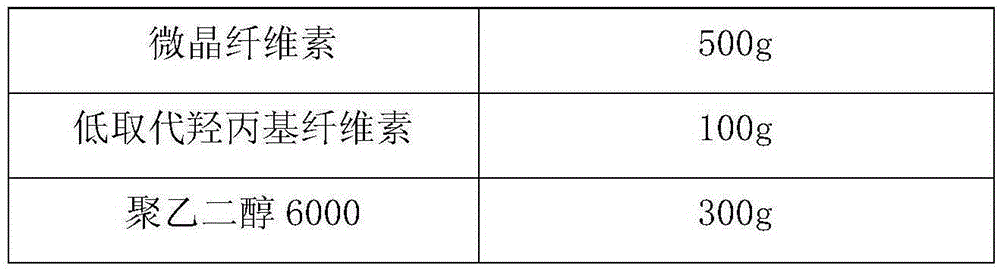
[0032]
[0033]
[0034] Preparation Process:
[0035] 1) Put 978.75g of clopidogrel hydrogen sulfate in a fluidized bed granulator and preheat to 45°C;
[0036] 2) Dissolving 300g polyethylene glycol 6000 in 50% ethanol to obtain a polyethylene glycol solution;
[0037] 3) dissolving polyethylene glycol in clopidogrel bisulfate sprayed into a boiling state, and drying to obtain granules;
[0038] 4) Pass the granules through a 20-mesh sieve for sizing, add 1000g of mannitol, 500g of microcrystalline cellulose and 100g of low-substituted hydroxypropyl cellulose to the sized granules, mix evenly, and press into tablets.
Embodiment 2
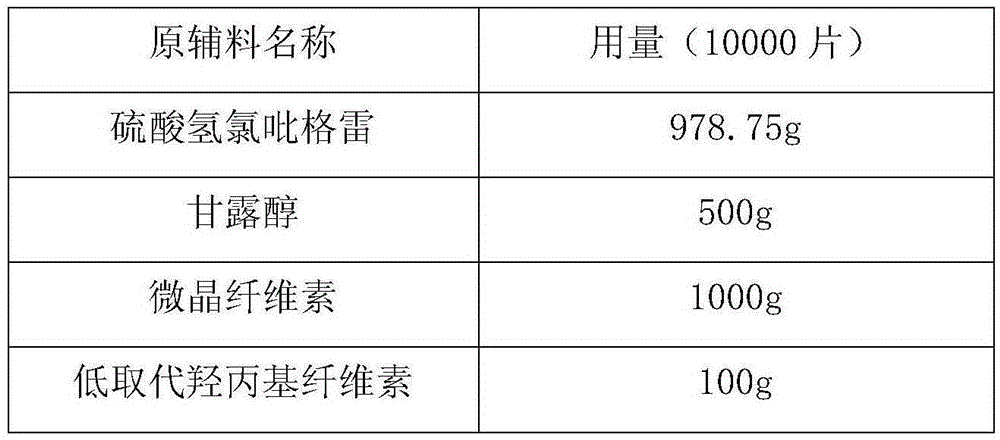
[0041] Preparation Process:
[0042] 1) Put 978.75g of clopidogrel bisulfate, 1000g of mannitol, 500g of microcrystalline cellulose and 100g of low-substituted hydroxypropyl cellulose in a fluidized bed granulator, mix well and preheat to 45°C;
[0043] 2) Dissolving 500g polyethylene glycol 6000 in 50% ethanol to obtain a polyethylene glycol solution;
[0044] 3) Polyethylene glycol is dissolved in the material sprayed into the boiling state, and after drying, granules are obtained;
[0045] 4) Pass the granules through a 20-mesh sieve for sizing, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com