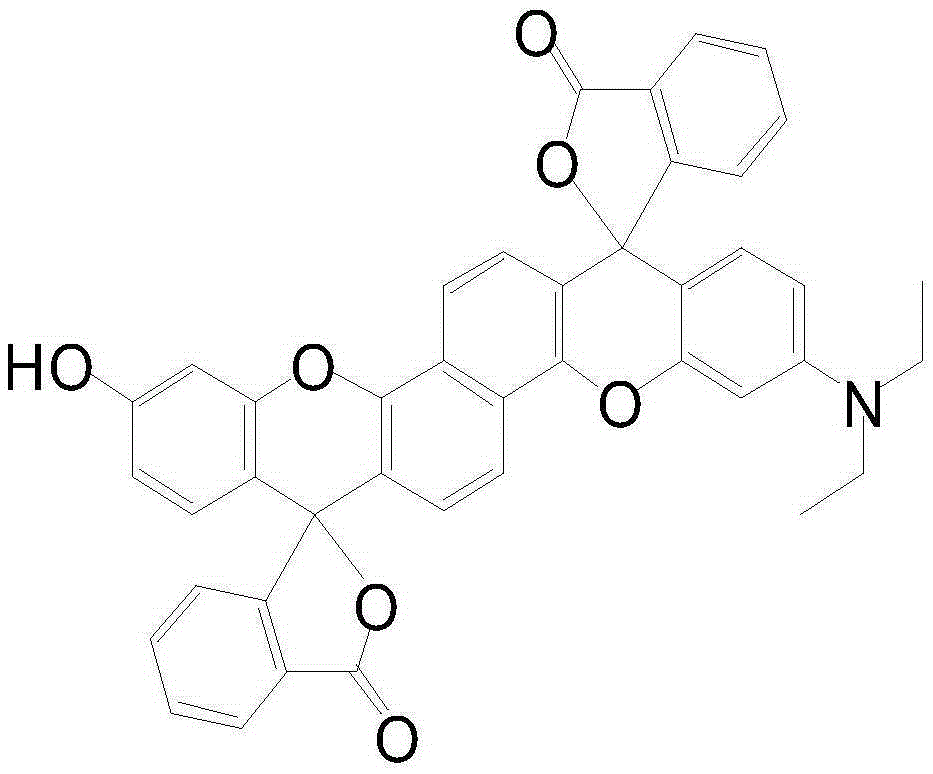
Preparation method of fluorescein and rhodamine structures-fused optical signal controllable dark red xanthene dye
An optical signal and fluorescein technology, which is applied in the field of the preparation of molecular fluorescent dyes, can solve the problems of poor resistance to photobleaching, inability to reach deep red or near-infrared bands, and limited wide application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1, intermediate product 1, concrete method is as follows:
[0032] The preparation steps of xanthene intermediate product 1 are as follows:
[0033]
[0034] 1) Add 2-(2,4-dihydroxybenzoyl)benzoic acid (0.492g, 2mmol) and 1,5-dihydroxynaphthalene (0.427g, 2.6mmol) into 5mL methanesulfonic acid and stir, oil at 85°C The reaction was heated in the bath for 6-8h, and the product solution was obtained after cooling.
[0035] 2) Add 60 mL of water to the reaction solution obtained in the previous step, and add an appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with hot ethyl acetate to collect the organic phase, and washed with anhydrous Na 2 SO 4 After drying and filtering, the obtained solid crude product was separated by column chromatography through a silica gel column with dichloromethane and ethanol to obtain intermediate product 1, 0.471 g of powdery white solid powder, and the yield was 62%. Me...
Embodiment 2
[0037] Embodiment 2, the preparation of xanthene fluorescent dye molecule 2, the specific method is as follows:
[0038] The synthesis steps are as follows:
[0039]
[0040] 1) The intermediate product 1 (0.224g, 0.5mmol) obtained in the above-mentioned Example 1 and 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (0.203g, 0.65mmol) were added to 5mL concentrated H 2 SO 4 Stir in medium, heat the reaction in an oil bath at 83°C for 6h, and obtain a product solution after cooling.
[0041] 2) Add 60 mL of water to the reaction solution obtained in the previous step, and add an appropriate amount of NaHCO 3 The solid neutralized the reaction solution, extracted with dichloromethane, collected the organic phase, and washed with anhydrous Na 2 SO 4 After drying and filtering, the obtained solid crude product was separated by column chromatography through a silica gel column with dichloromethane and ethanol to obtain xanthene fluorescent dye 2, 0.131 g of powdery white soli...
Embodiment 3
[0043] Embodiment 3, the determination of the optical properties of the xanthene dye 2:
[0044] The dye compound 2 was made into a concentration of 5×10 -3 mol / L DMF solution, keep away from light for future use.
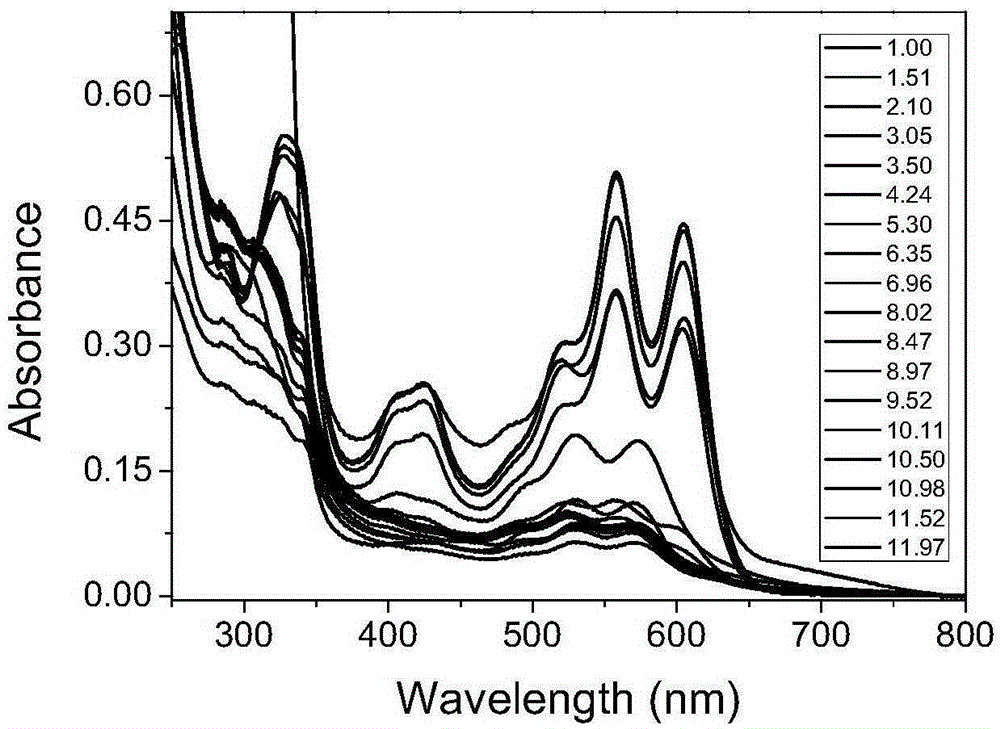
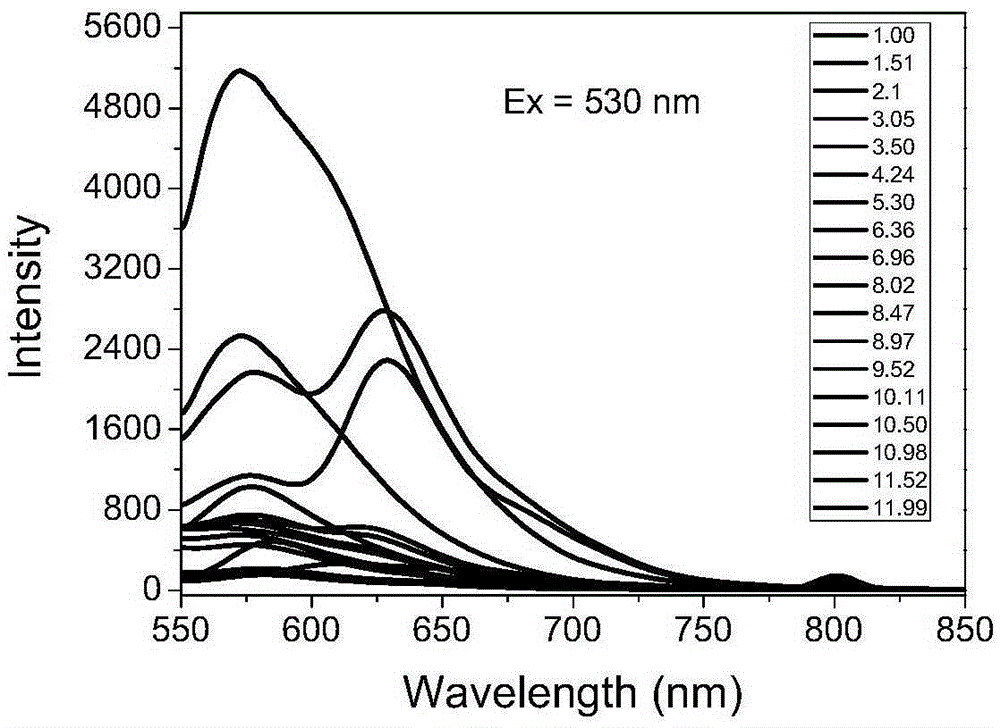
[0045] 1) Determination of the optical properties of compound 2 in different solvents: Compound 2 was prepared in different solvents to 5×10 -6 mol / L of the liquid to be tested was 3mL, and its ultraviolet absorption spectrum and fluorescence emission spectrum were measured before and after adding 1% trifluoroacetic acid. The dyestuff was stable in the structure of spirolide in almost all organic solvents, but for Acid sensitive.
[0046] 2) Determination of the influence of pH on absorption spectrum or fluorescence spectrum: the dye compound 2 was prepared into 5×10 under different pH (1-14) conditions -6 mol / L of the test solution 3mL. The ultraviolet-visible absorption and emission spectra were measured at room temperature. The results are attached figure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com