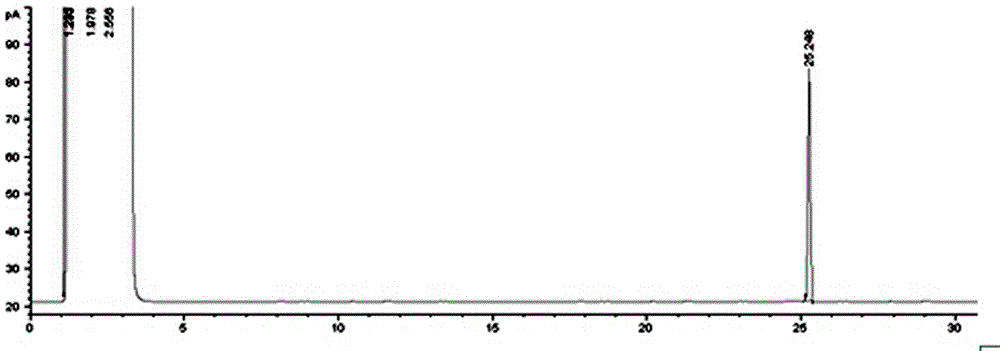
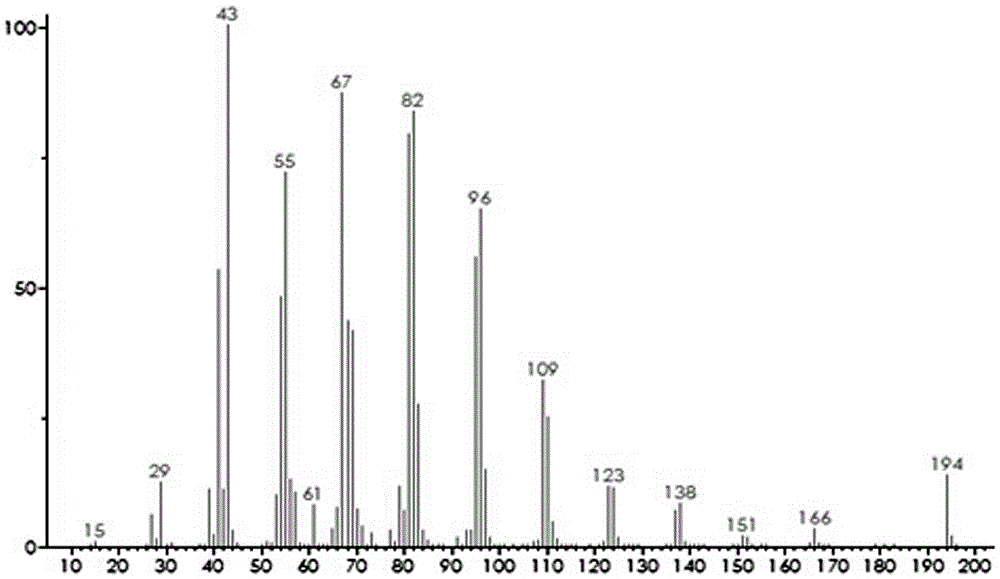
Method for synthesizing cis-7-tetradecenol acetate which is main component of sex pheromone of holcocerus vicarius walker
A technology of carbenol acetate and tetradecenol, which is applied in the synthesis field of acetates, can solve the problems of low yield, dangerous production process and high synthesis cost, and achieves high product yield, low cost, Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A method for synthesizing cis-7-tetradecenyl acetate, the main component of the sex pheromone of the elm beetle moth, comprises the following steps:
[0018] (1) Preparation of (7-bromoheptyl-1-)-2-tetrahydropyranyl ether In a 250mL four-neck flask equipped with an electromagnetic stirrer, a reflux condenser, and a constant pressure dropping funnel, add 3, 4-dihydropyran 8g, under stirring, add 0.03g of 37% hydrochloric acid as a catalyst, start to drop 17g of 7-bromo-1-heptanol after 10min, control the rate of addition (this reaction is an exothermic reaction, 7-bromo When the drop rate of Dai-1-heptanol is accelerated, the reaction will be accelerated and the exotherm will increase, so it must be cooled in an ice bath), keep the temperature of the reaction system at 40-60°C, react at room temperature for 2 hours after the addition, stop stirring, and change to a distillation device, Unreacted 3,4-dihydropyran was distilled off. After the residual liquid was cooled, 8...
Embodiment 2
[0024] (1) Preparation of (7-bromoheptyl-1-)-2-tetrahydropyranyl ether In a 500mL four-neck flask equipped with an electromagnetic stirrer, a reflux condenser, and a constant pressure dropping funnel, add 3, 20g of 4-dihydropyran, add 0.06g of 37% hydrochloric acid as a catalyst under stirring, start to drop 34g of 7-bromo-1-heptanol after 10min, control the rate of addition (this reaction is an exothermic reaction, 7-bromo When the drop rate of Dai-1-heptanol is accelerated, the reaction will be accelerated and the exotherm will increase, so it must be cooled in an ice bath), keep the temperature of the reaction system at 40-60°C, react at room temperature for 2 hours after the addition, stop stirring, and change to a distillation device, Unreacted 3,4-dihydropyran was distilled off. After the residual liquid was cooled, 170 g of petroleum ether was added, washed with saturated sodium bicarbonate solution, saturated brine, dried over sodium sulfate for 10-15 hours, concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com