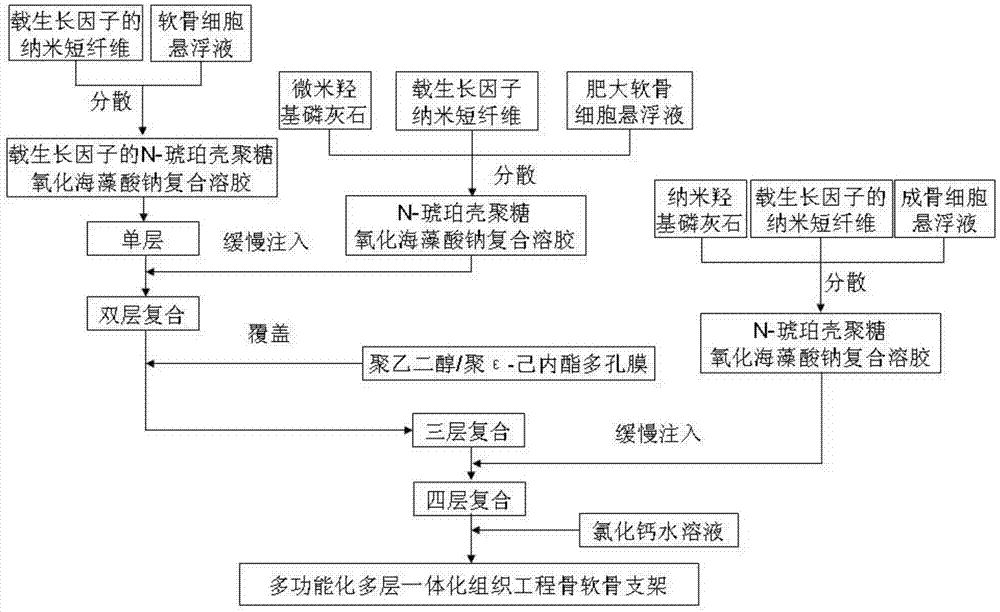
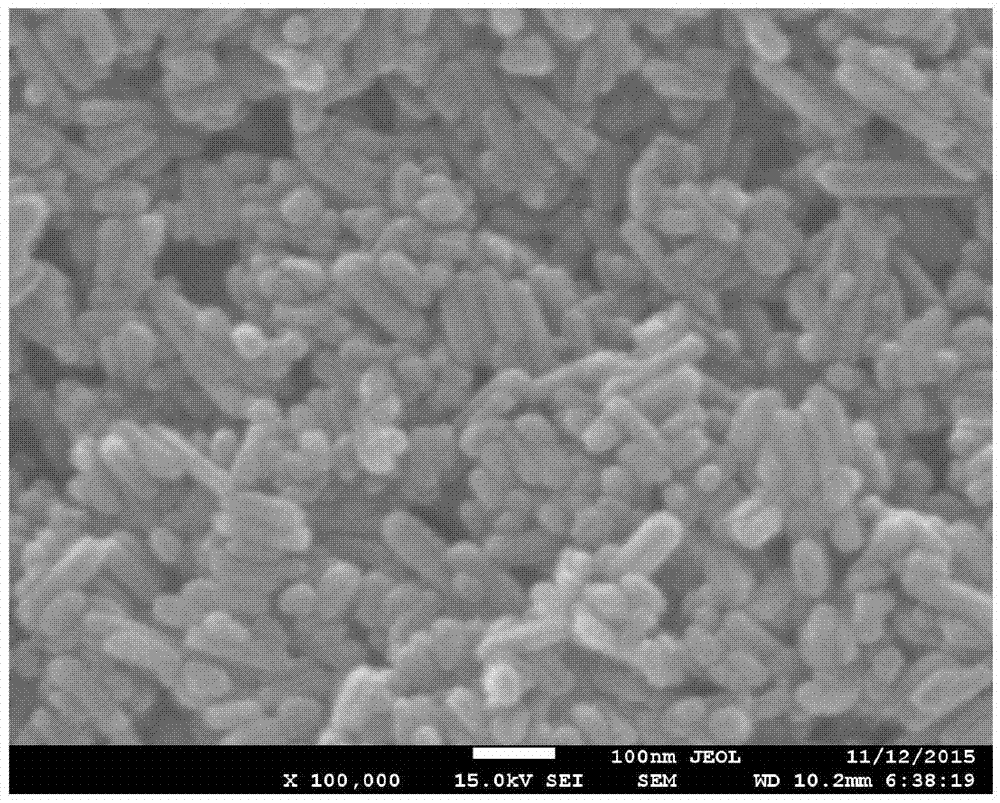
Tissue-engineered bone cartilage composite scaffold and preparation method thereof
A tissue-engineered bone and composite scaffold technology, used in tissue regeneration, medical science, prosthesis, etc., to achieve the effects of good biocompatibility, biocompatibility, degradability, and enhanced mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Accurately weigh 1.00g of polyε-caprolactone, add it into 5mL of chloroform, and prepare a 20w / v% solution, stir and dissolve evenly; accurately weigh 2.00g of polyethylene glycol, add 50ngBMP-2 into 5mL of distilled water, and prepare 40w / v% solution, magnetic stirring to dissolve evenly. Inject into 5mL dry syringes respectively, the syringes are fixed in the card slot of the micro-injection pump, and the output end of the high-voltage DC power supply is fixed on the coaxial nozzle (the inner diameter of the outer needle is 1.1mm, the outer diameter of the inner needle is 0.6mm, and the inner diameter of the inner needle is 0.3 mm), the outer axis is polyε-caprolactone solution, and the flow rate is set at 0.5mL / h. The inner axis is polyethylene glycol solution, the flow rate is set to 0.1mL / h, and the DC high voltage is applied to 20kV. The high-speed rotating drum is used as the receiver (the speed is 1200r / min), and the distance between the coaxial nozzle and the r...
Embodiment 2
[0049] Accurately weigh 1.00g of polyε-caprolactone, add it into 5mL of chloroform, and prepare a 20w / v% solution, stir and dissolve evenly; accurately weigh 2.00g of polyethylene glycol, add 50ngBMP-2 into 5mL of distilled water, and prepare 40w / v% solution, magnetic stirring to dissolve evenly. Inject into 5mL dry syringes respectively, the syringes are fixed in the card slot of the micro-injection pump, and the output end of the high-voltage DC power supply is fixed on the coaxial nozzle (the inner diameter of the outer needle is 1.1mm, the outer diameter of the inner needle is 0.6mm, and the inner diameter of the inner needle is 0.3 mm), the outer axis is polyε-caprolactone solution, and the flow rate is set to 0.5mL / h; the inner axis is polyethylene glycol solution, the flow rate is set to 0.1mL / h, and the DC high voltage is 20kV, which is rotated at a high speed. The roller is used as a receiver (rotating speed is 1200r / min), and the distance between the coaxial nozzle a...
Embodiment 3
[0057] Accurately weigh 1.00g of polyε-caprolactone, add it into 5mL of chloroform, and prepare a 20w / v% solution, stir and dissolve evenly; accurately weigh 2.00g of polyethylene glycol, add 50ngBMP-2 into 5mL of distilled water, and prepare 40w / v% solution, magnetic stirring to dissolve evenly. Inject into 5mL dry syringes respectively, the syringes are fixed in the card slot of the micro-injection pump, and the output end of the high-voltage DC power supply is fixed on the coaxial nozzle (the inner diameter of the outer needle is 1.1mm, the outer diameter of the inner needle is 0.6mm, and the inner diameter of the inner needle is 0.3 mm), the outer axis is polyε-caprolactone solution, and the flow rate is set to 0.5mL / h; the inner axis is polyethylene glycol solution, the flow rate is set to 0.1mL / h, and the DC high voltage is 20kV, which is rotated at a high speed. The roller is used as a receiver (rotating speed is 1200r / min), and the distance between the coaxial nozzle a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com