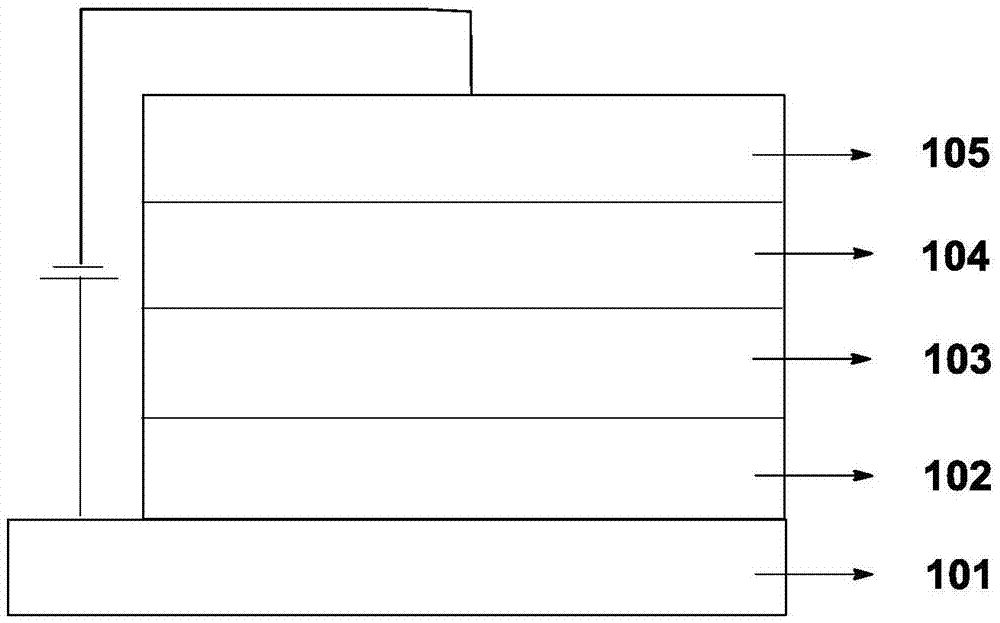
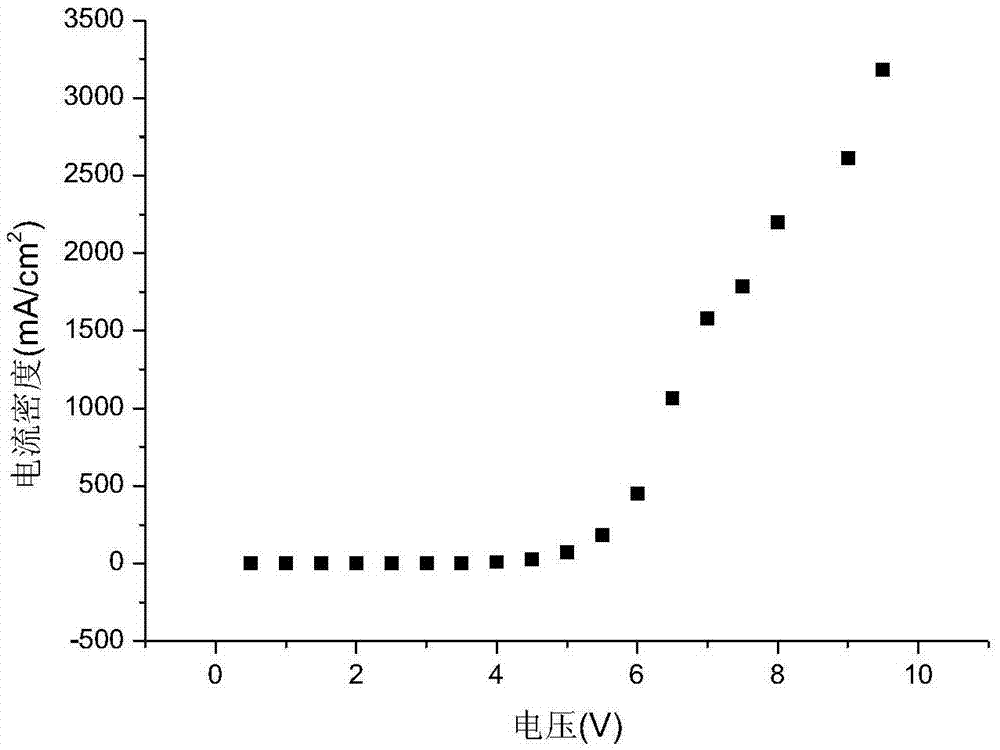
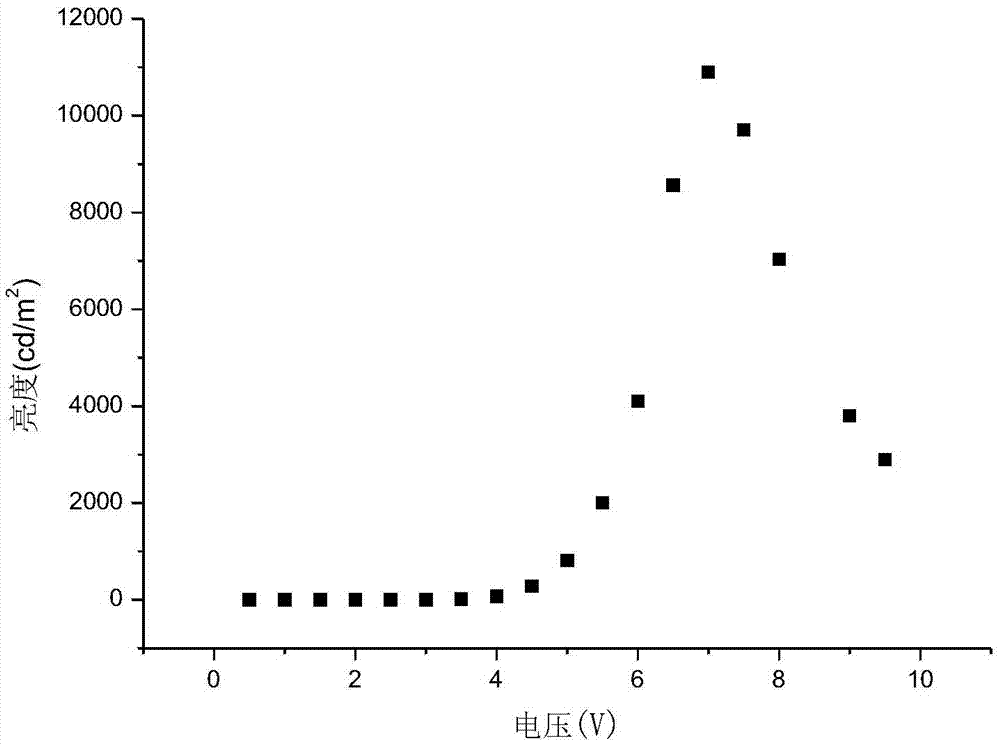
Organic electroluminescent material and application thereof
An electroluminescent material and luminescent technology, applied in luminescent materials, organic chemistry, circuits, etc., can solve problems such as poor film stability, achieve good film stability and thermal stability, large steric hindrance, and start-up Effect of Brightness Voltage Reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 intermediate 3
[0039]
[0040] Preparation of Compound 1: In a 2L three-necked flask, add 8-quinolineboronic acid (72.6g, 0.42mol), methyl o-bromobenzoate (86.0g, 0.40mol), potassium carbonate (82.8g, 0.6mol), Pd (PPh 3 ) 4 (2.31g, 2mmol), toluene (800mL), N 2 Protect, heat up to reflux, keep warm for 2 hours, stop the reaction, cool down to 25°C, wash with water and separate the liquid, collect the organic phase, remove the solvent to obtain the crude compound 1 as a yellow viscous oil, and directly proceed to the next step without purification ( Yields are based on 100%).
[0041] Preparation of Compound 2: Add 833 mL of methyllithium solution (1.0 mol) into a 2L three-necked flask, and cool down to -78°C in a low-temperature bath under nitrogen protection. The crude product of Compound 1 is dissolved in tetrahydrofuran (400 g) and transferred to a constant temperature In the pressure funnel, add dropwise to the above-mentioned...
Embodiment 2
[0045] The preparation of embodiment 2 compound C01
[0046]
[0047] In a 100mL three-necked flask, add compound 3 (4.03g, 10mmol) prepared in Example 1, diphenylamine (3.72g, 22mmol), sodium tert-butoxide (2.88g, 30mmol), palladium acetate (0.01g, 0.04mmol) , tri-tert-butylphosphine (0.016g, 0.08mmol), xylene (50mL), N 2 Protect, heat up to reflux, keep warm for 8 hours, stop the reaction, cool down to 25°C, add 30mL of deionized water, stir for 5min, separate liquids, collect the organic phase, remove the solvent to obtain the crude product of target C01, use silica gel column layer Analysis and purification, the eluent is petroleum ether: dichloromethane=3:1 (V / V), after removing the solvent, use dichloroethane as solvent recrystallization to obtain the target object C01, off-white solid 4.5g, using chemical The vapor phase deposition system was further sublimated and purified, and the sublimation temperature was 330° C. to obtain 3.6 g of compound C01 with a yield of ...
Embodiment 3
[0049] The preparation of embodiment 3 compound C02
[0050]
[0051] Referring to Example 2, the raw materials were compound 3 and N-phenyl-1-naphthylamine prepared in Example 1 to obtain compound C02 with a yield of 65.6%. High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 50 h 37 N 3 , the theoretical value is 679.2987, and the test value is 679.2982. Elemental analysis (C 50 h 37 N 3 ), theoretical value C: 88.33, H: 5.49, N: 6.18, measured value C: 88.37, H: 5.47, N: 6.16.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com