Sucrose isomerase mutant and method for producing isomaltulose
A technology of sucrose isomerase and isomaltulose, which is applied in the field of separation of immobilized enzymes, achieves the effects of saving economic costs, easy implementation of enzyme production efficiency, and reduced operation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Cloning and expression of sucrose isomerase gene in Bacillus licheniformis
[0047] 1. Construction of recombinant strains
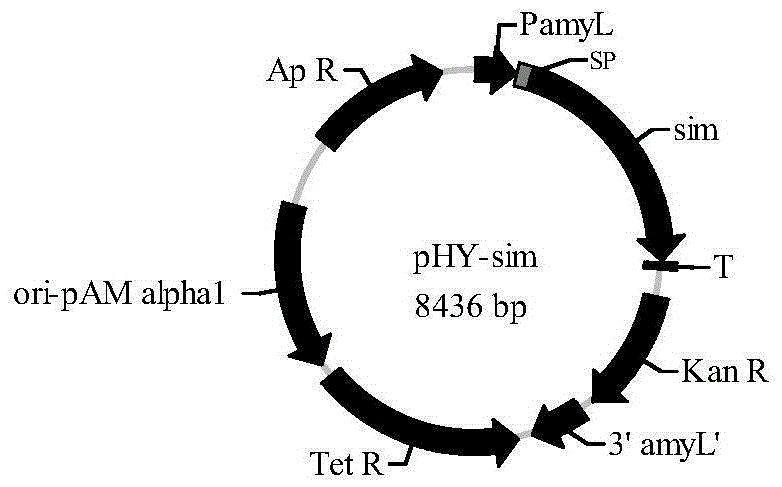
[0048] Using the genomic DNA of Pantoeadispersa UQ68J as a template, primers were designed according to the sequence of the sucrose isomerase gene sim (GenBank accession number: AY223549.1) of P. SEQ ID NO: 14) and R1 (SEQ ID NO: 15) amplify the sim gene (nucleotide sequence SEQ ID NO: 1) that does not contain a signal peptide, see SEQ ID NO: 2 for the amino acid sequence. PCR amplification system and reaction conditions reference HSDNA Polymerase instruction sheet (TaKaRa). The PCR product was cloned into the plasmid pMD18-Tsimple to obtain the recombinant plasmid pMD18-T-sim with the sim gene. After double digestion with XbaI and SmaI, the recombinant plasmid was cloned into the corresponding site of the expression vector pHY-WZX (DandanNiuandZhengxiangWang. JIndMicrobiolBiotechnol, 2007, 34:357-362) to obtain the recombinant expr...
Embodiment 2
[0062] Example 2: Construction and screening of sucrose isomerase mutants
[0063] Taking Tyr307Asp as an example, using the plasmid pMD18-T-sim as a template, primers F1 (SEQ ID NO: 15) and R2 (SEQ ID NO: 18) were used to amplify by conventional PCR method to obtain PCR product 1. Then use primers F2 (SEQ ID NO: 17) and R1 (SEQ ID NO: 16) to amplify by conventional PCR method to obtain PCR product 2.
[0064] The above two reactions are carried out synchronously. The two amplification products, PCR product 1 and PCR product 2, are purified and recovered, mixed equimolarly and added to the second-step reaction system. No primers are added to the reaction system, and the others are the same as conventional PCR. The reaction system is circulated for 5 to 10 times.
[0065] Using the amplified product of the second-step reaction system as a template, add primers F1 and R1, and perform 25 to 30 cycles in the same way as the conventional PCR reaction system. The amplified product...
Embodiment 3
[0073] Example 3: Enzyme activity and catalytic product analysis of sucrose isomerase mutants
[0074] The enzyme activity assay was carried out according to the method of Example 1, and the specific results are shown in Table 3. It can be seen from the table that the mutation did not cause a significant decrease in enzyme activity, and all 11 mutants maintained good biological activity.
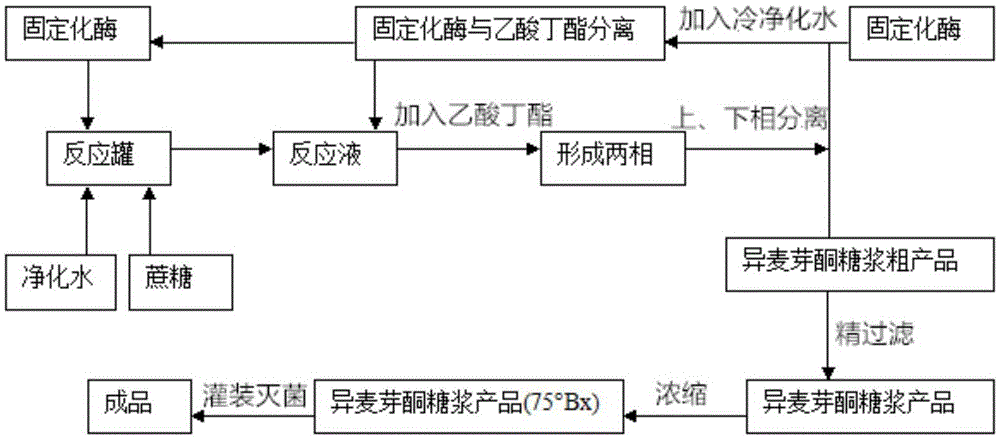
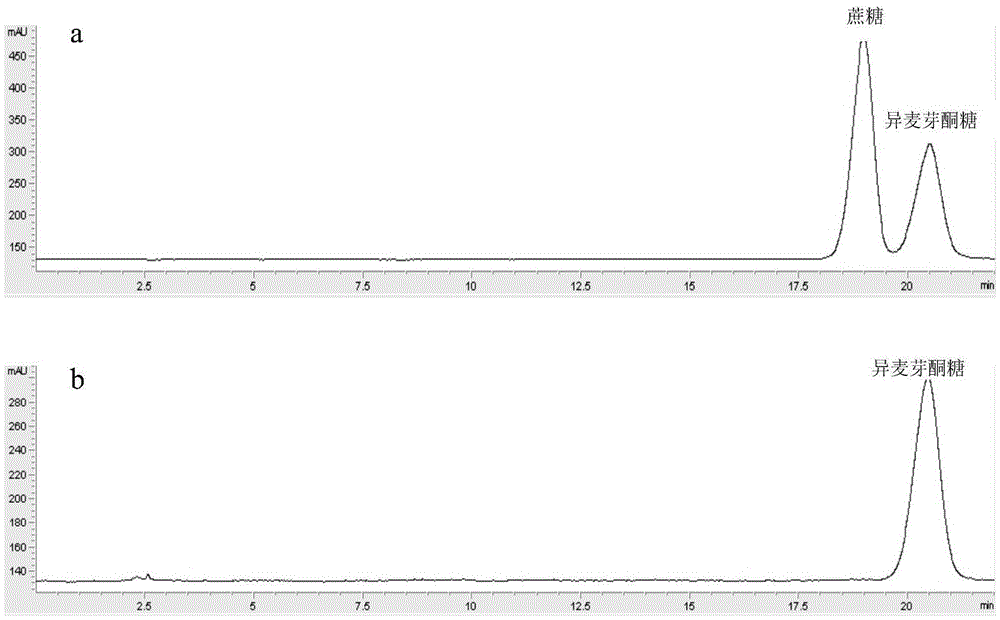
[0075] With 10 mL of sucrose at a concentration of 50% as the substrate, the fermentation liquid of the sucrose isomerase mutant was added at 30°C, and the reaction time was extended to 100 min to complete the conversion of sucrose and facilitate the analysis of the product ratio. The obtained reaction liquid was diluted 10 times, Detection by HPLC method. Taking the recombinant bacterium CBBD302 (pHY-sim) obtained in Example 1 as a control, the results are shown in Table 3. Mutation of Gln to Glu at position 310, a negatively charged acidic amino acid mutated into an uncharged polar amino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com