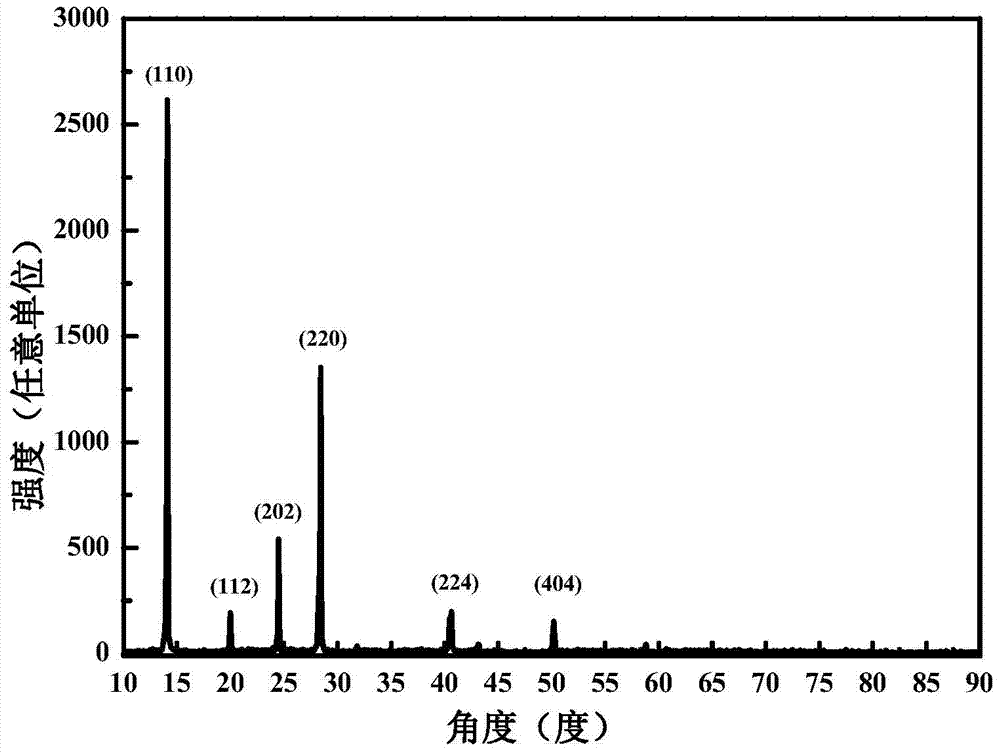
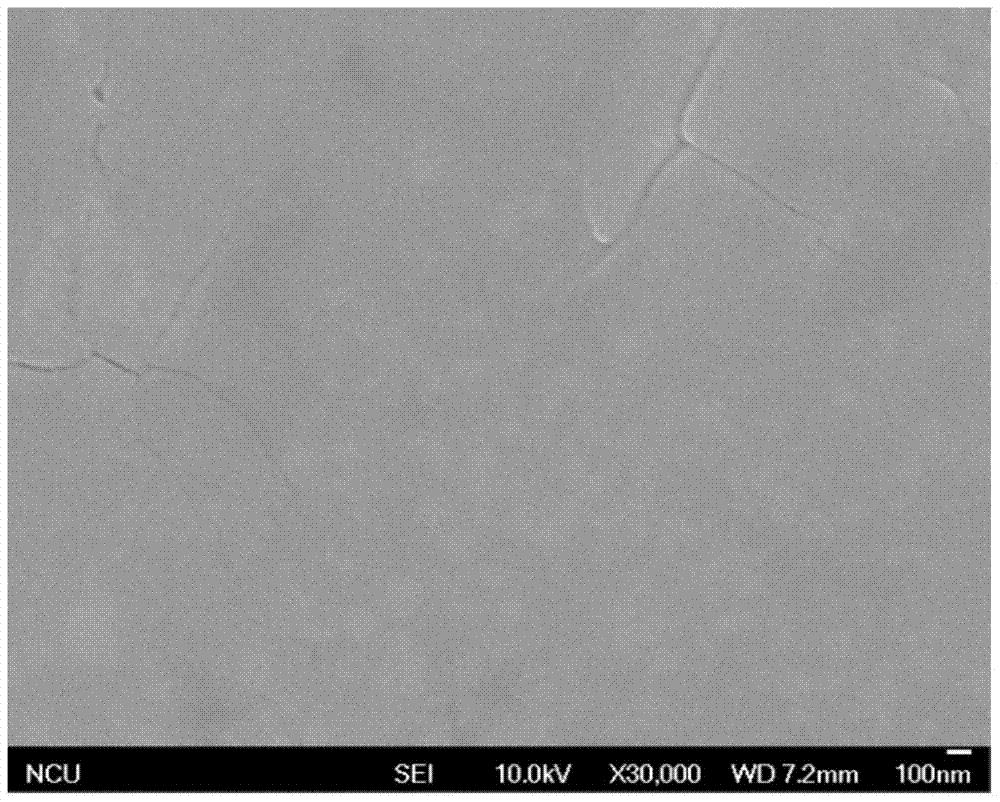
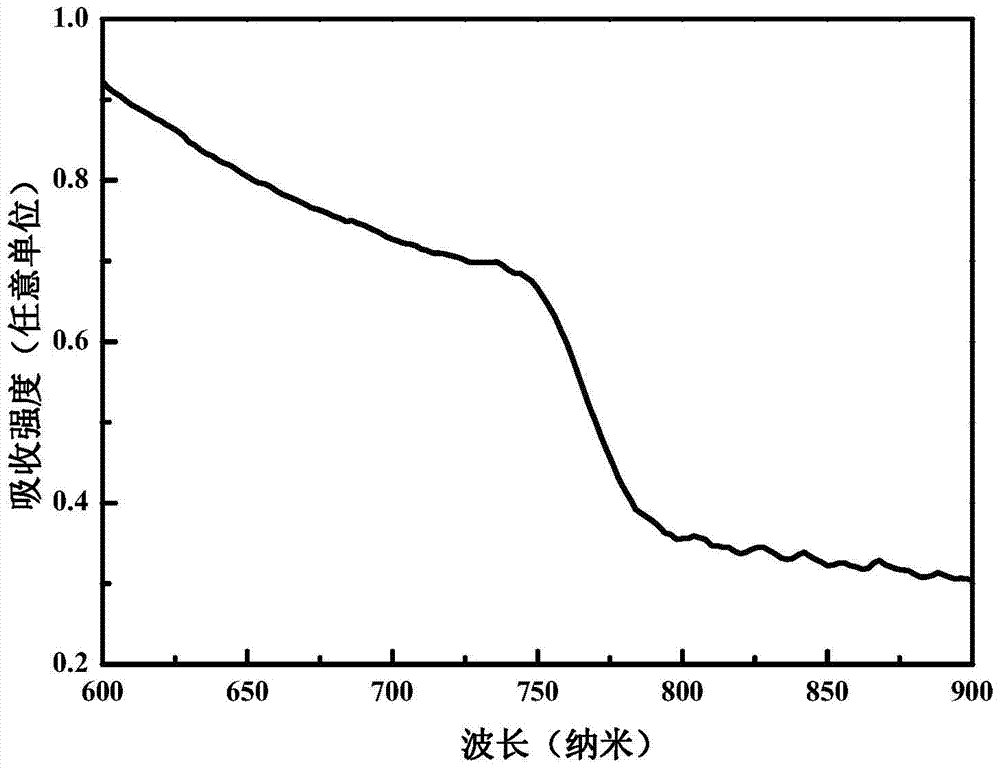
A method for preparing methylamine lead iodine perovskite thin films based on lead xanthate complexes
A methylamine lead iodine complex technology is applied in the field of preparation of methylamine lead iodide perovskite thin films, and can solve the problem of difficult control of grain nucleation and growth process, poor film continuity, uniformity and reproducibility, and influence of The problems of improving the efficiency of perovskite thin-film solar cells, and achieving the effects of easy control of preparation parameters, high photoelectric conversion efficiency, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 0.6412 g of potassium ethyl xanthate in 50 ml of deionized water, dissolve 0.7587 g of lead acetate in 400 ml of deionized water, add the lead acetate solution to the potassium ethyl xanthate solution, and stir at room temperature for 3 hours to obtain precipitation. Thereafter, suction was filtered, washed, and the resulting precipitate was vacuum-dried overnight. Then dissolve the precipitate in dichloromethane, add pyridine (the molar ratio of the precipitate to pyridine is 1:2), stir at room temperature for 30 minutes, and after the precipitate is completely dissolved in the solution to form a homogeneous solution, use a rotary evaporator to remove excess solvent to obtain a white powder. Dissolve the white powder in acetone, filter with suction, take the filtrate and spin evaporate the solvent, add a small amount of ethanol to wash off impurities, and filter with suction to obtain lead ethyl xanthate complex. Lead ethyl xanthate complex and CH 3 NH 3I ...
Embodiment 2
[0032] Dissolve 0.6890g of sodium n-butylxanthate in 50ml of deionized water, dissolve 0.7587g of lead acetate in 400ml of deionized water, add the lead acetate solution to the solution of sodium n-butylxanthate, stir at room temperature for 3 hours to precipitate. Thereafter, suction was filtered, washed, and the resulting precipitate was vacuum-dried overnight. Then dissolve the precipitate in dichloromethane, add pyridine (the molar ratio of the precipitate to pyridine is 1:2), stir at room temperature for 30 minutes, and after the precipitate is completely dissolved in the solution to form a homogeneous solution, use a rotary evaporator to remove excess solvent to obtain a white powder. Dissolve the white powder in acetone, filter with suction, take the filtrate and spin evaporate the solvent, add a small amount of ethanol to wash off impurities, and filter with suction to obtain lead ethyl xanthate complex. Lead ethyl xanthate complex and CH 3 NH 3 I was dissolved in ...
example 3
[0035] Dissolve 0.6973g of potassium isopropylxanthate in 50ml of deionized water, dissolve 1.0282g of lead nitrate in 400ml of deionized water, add the lead acetate solution to the potassium isopropylxanthate solution, and stir at room temperature A precipitate was obtained in 3 hours. Thereafter, suction was filtered, washed, and the resulting precipitate was vacuum-dried overnight. Then dissolve the precipitate in dichloromethane, add pyridine (the molar ratio of the precipitate to pyridine is 1:2), stir at room temperature for 30 minutes, and after the precipitate is completely dissolved in the solution to form a homogeneous solution, use a rotary evaporator to remove excess solvent to obtain a white powder. Dissolve the white powder in acetone, filter with suction, take the filtrate and spin evaporate the solvent, add a small amount of ethanol to wash off impurities, and filter with suction to obtain lead ethyl xanthate complex. Lead ethyl xanthate complex and CH 3 NH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com