Rabies virus nucleoprotein monoclonal antibody and application thereof
A technology of monoclonal antibody and rabies virus, which is applied in the field of viral protein immune analysis, can solve problems that plague human beings, and achieve high sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The establishment of embodiment 1 hybridoma cell line
[0027] 1. Experimental materials
[0028] 1. Immunogen: The rabies virus SRV9 strain was used as the immunogen.
[0029] 2. Culture medium: DMEM medium was purchased from Hyclone Company; HAT, HT selection medium and pristane were purchased from Sigma Company.
[0030] 3. Experimental animals: Balb / c mice, 8-12 weeks old, female, SPF grade animal culture.
[0031] 4. Other materials: Freund's complete adjuvant and Freund's incomplete adjuvant were purchased from Sigma Company; PEG4000 was purchased from Fluka Company; HRP-goat anti-mouse IgG antibody was purchased from JacksonImmune Company; other reagents were domestic analytically pure products.
[0032] 2. Establishment of hybridoma cell lines
[0033] 1. Animal immunity
[0034] 1) Basic immunization: The immunogen was mixed with Freund's complete adjuvant in equal volumes and fully emulsified, and injected subcutaneously in points, each injection volume of...
Embodiment 2
[0050] The preparation of the monoclonal antibody of embodiment 2 anti-rabies virus nucleoproteins
[0051] 1. Antibody preparation
[0052] Adult BALB / c mice were selected, and pristane was inoculated intraperitoneally, 0.5ml per mouse. After 7-10 days, the 16th generation cells of the two strains of hybridoma cells in Example 1 were inoculated into the peritoneal cavity respectively, and each mouse was 1×10 6 -2×10 6 indivual. After an interval of 5 days, when the abdomen is obviously enlarged and the skin feels tense when touched with hands, the ascites can be collected with a No. 9 needle.
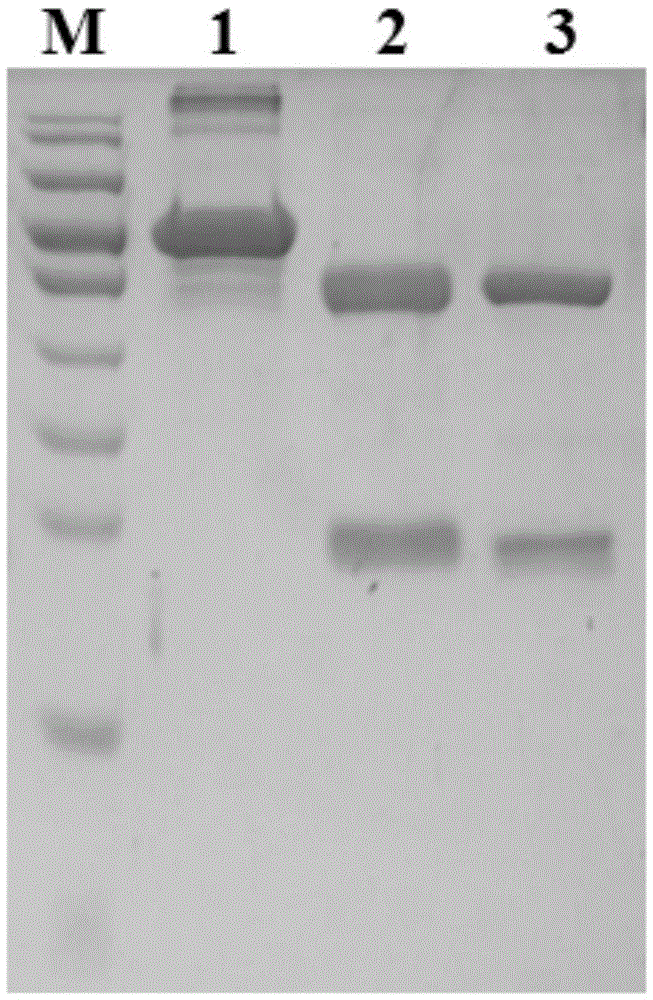
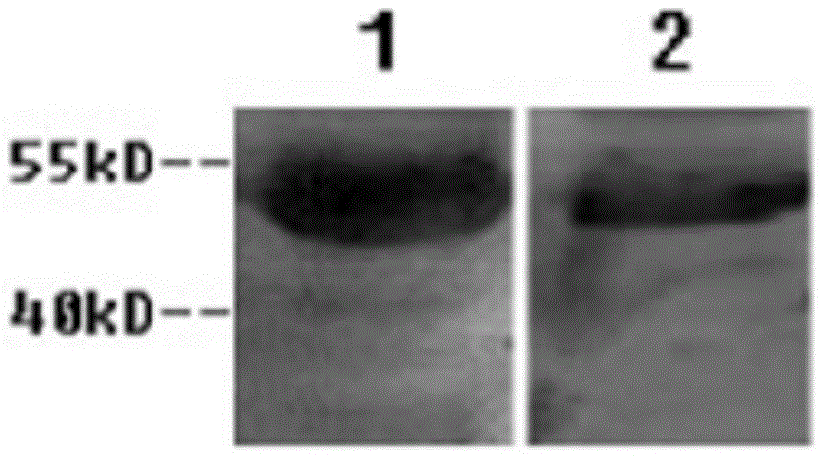
[0053] Centrifuge the ascitic fluid (13000r / min for 30 minutes), remove cell components and other precipitates, and collect the supernatant. Purify with ProteinG~SepharoseCL-4B, the upper column liquid is 20mM PBS buffer, and the column chromatography eluent is: pH 2.7, 20mM glycine buffer, respectively, to obtain monoclonal antibodies against rabies virus, which specifically recog...
Embodiment 3
[0063] Example 3 Application of Purified Antibody to Prepare Anti-rabies Virus Detection Reagent
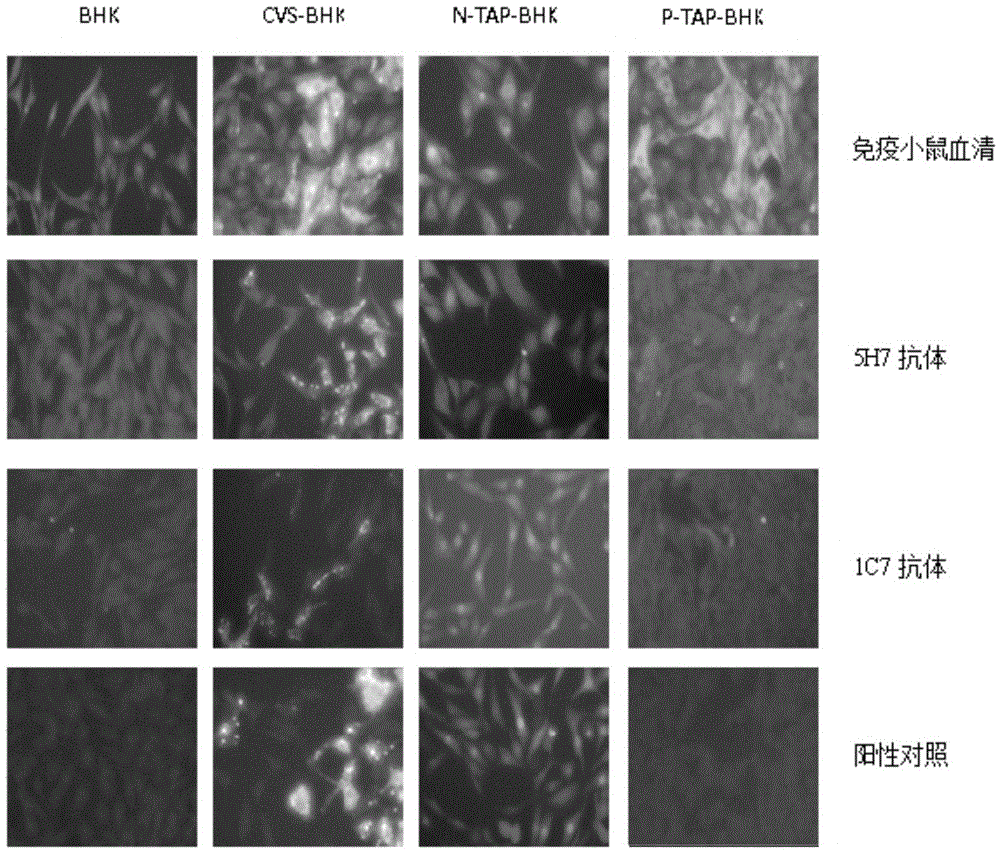
[0064] ELISA double-antibody sandwich method: 5H7 and 1C7 antibodies were used for pairing experiments, and 5H7 was determined as the coating antibody, and HRP-labeled 1C7 was used as the detection antibody. The ELISA detection method was determined. The detection sensitivity of the kit can reach 0.003125IU / ml. The results are shown in Table 3, and the fitting curve formulated according to the results is as follows Figure 4 shown. Antibodies were labeled with the modified sodium periodate method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com