Nucleic acid detection kit for human papillomavirus and method and application thereof
A technology for human papillomavirus and detection kits, which is applied in the directions of microorganism-based methods, biochemical equipment and methods, and microbial determination/inspection, etc. Inability to distinguish accurately, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0093] A human papillomavirus nucleic acid detection kit is provided in this embodiment, which can qualitatively detect the infection of high-risk types and low-risk types of human papillomaviruses (HPV), such as 16, 18, 52 . to test. The kit can be used for auxiliary diagnosis of clinical HPV infection and early screening of cervical cancer.
[0094] 1. Kit A: Storage temperature: 2-8°C
[0095] Table 3 Nucleic acid extraction reagents
[0096]
[0097] Wherein, the lysis solution is a solution containing guanidine isothiocyanate and TritonX-100, or the lysis solution is a solution containing guanidine isothiocyanate and Tween-20; wherein, the concentration of guanidine isothiocyanate in the lysis solution is 4M, the volume concentration of TritonX-100 or Tween-20 is 1.5%;
[0098] The preparation method of the binding solution is as follows: dissolve 200g of sodium perchlorate and 30g of sodium acetate in a small amount of purified water, then add 500ml of absolute et...
Embodiment 1
[0182] Example 1 Using the above kit to detect HPV infection in clinical samples
[0183] 100 positive clinical samples were tested with the above kit, and all samples were verified by the gold standard sequencing method. The test results and analysis results are shown in the table below.
[0184] Use the above kit to detect HPV infection in clinical samples.
[0185] Table 11
[0186]
[0187]
[0188]
[0189]
[0190]
[0191]
[0192]
[0193]
[0194] The comparison between the test results of the above kits and the sequencing results is shown in the table below:
[0195] Table 12
[0196]
[0197] Note: The clinical sample type not detected by the above kit is the HPV type outside the detection range of the kit.
[0198] The above kits were used to detect 100 positive clinical samples. For HPV types within the detection range (HPV16, 18, 52, 58, 31, 33, 45, 6, 11, 35, 39, 51, 53, 56, 59, 66, 68, 73, 82, 26, 40, 42, 43, 44) have good detection...
Embodiment 2
[0199] Example 2 The above kit was used to detect HPV infection in clinical mixed infection samples.
[0200] The collected 50 cases of mixed infection samples were tested separately, and the test results are as follows:
[0201] Table 13
[0202]
[0203]
[0204]
[0205] Among the 50 mixed infection samples, the detection results of the above kits were the same as those given by the sampling unit, and the HPV genotypes within the detection range could be accurately detected, and individual rare samples were not included in the collected samples.
[0206] In summary, the performance indicators of the kit in the present embodiment are as follows:
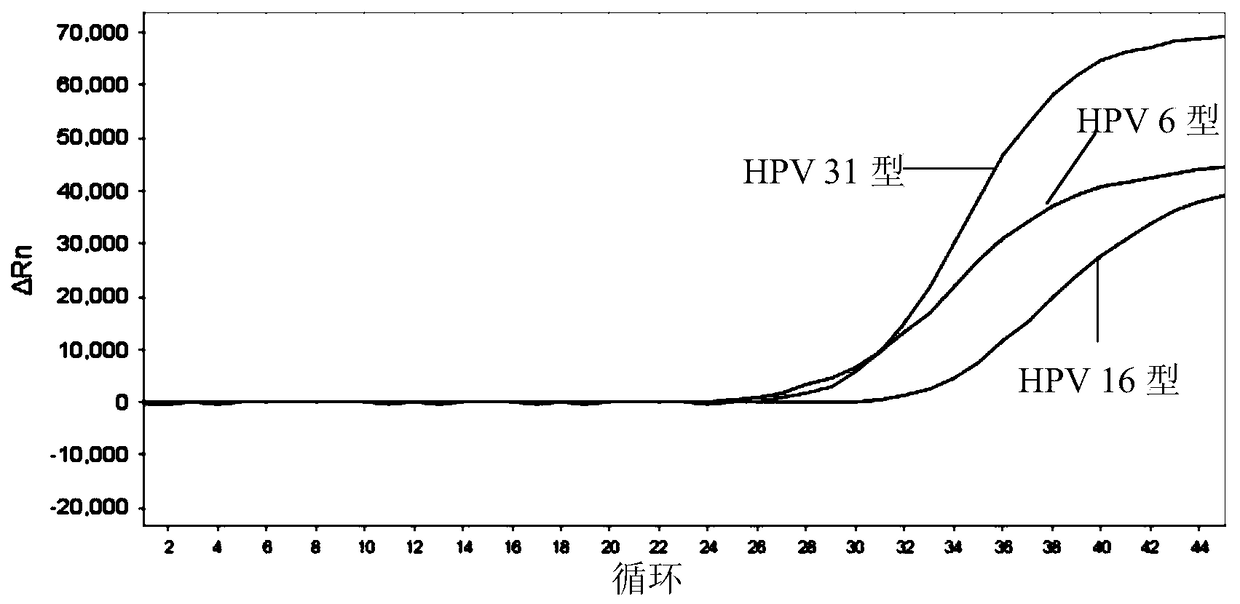
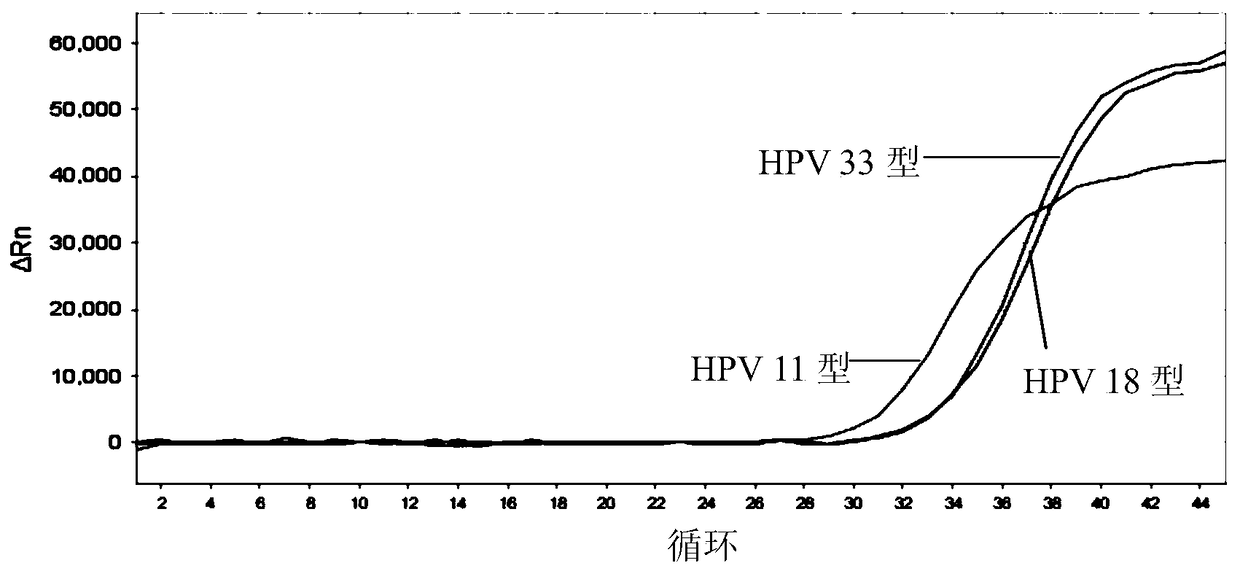
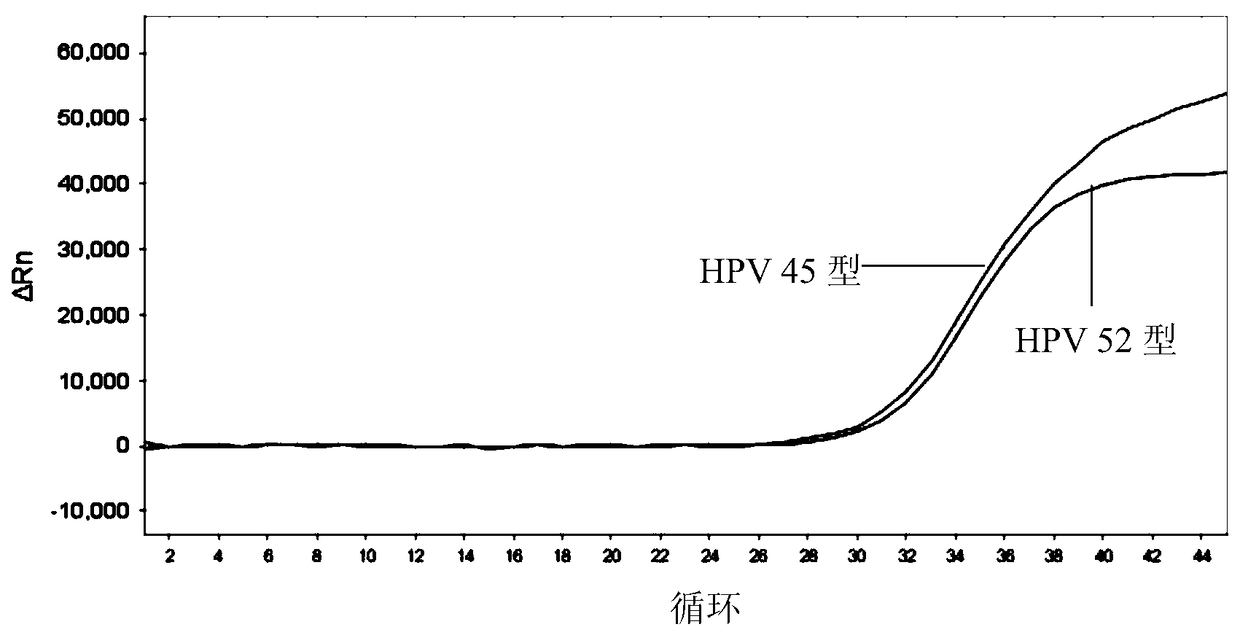
[0207] 1) Sensitivity: The minimum detection limit of HPV that can be stably detected by this kit is: 1.0×10 3 copies / ml. Figure 1-5 The detection limit of detection (LOD) of the above kits for each type of HPV is given.
[0208] 2) Genotype detection: this product can be used for 26, 31, 33, 35, 39, 52, 53, 56, 58, 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com