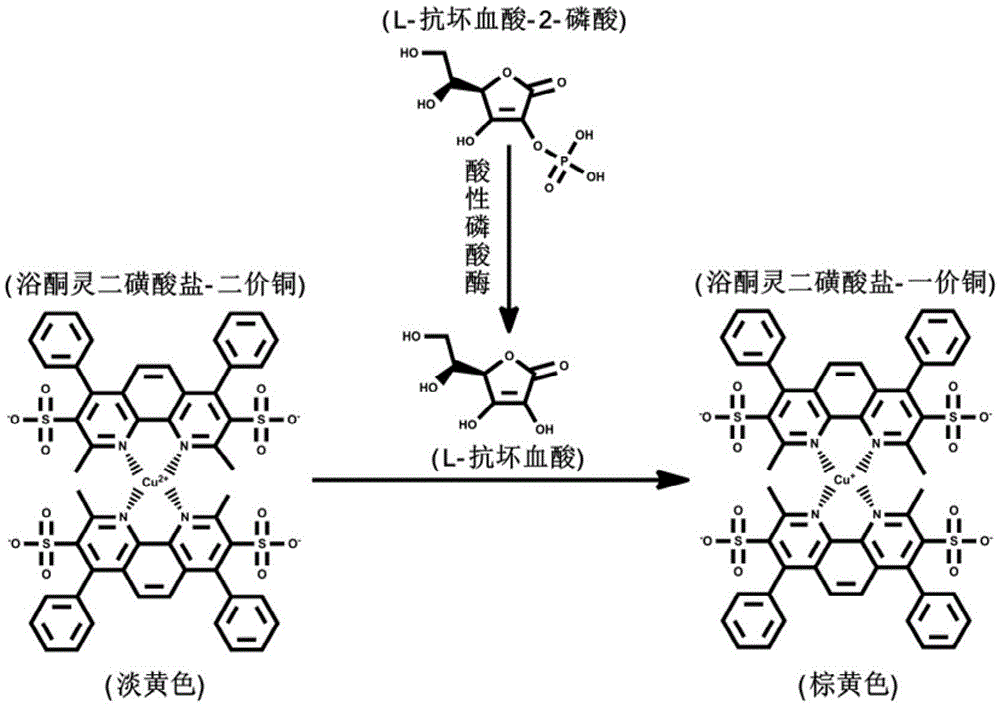
Measurement method of acid phosphatase activity
An acid phosphatase and assay method technology, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve the problems of poor anti-interference ability, cumbersome detection process, low sensitivity, etc., and achieves strong anti-interference ability and sensitivity. The effect of high and high sensitivity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Feasibility analysis of colorimetric analysis of acid phosphatase activity by the method of the present invention.
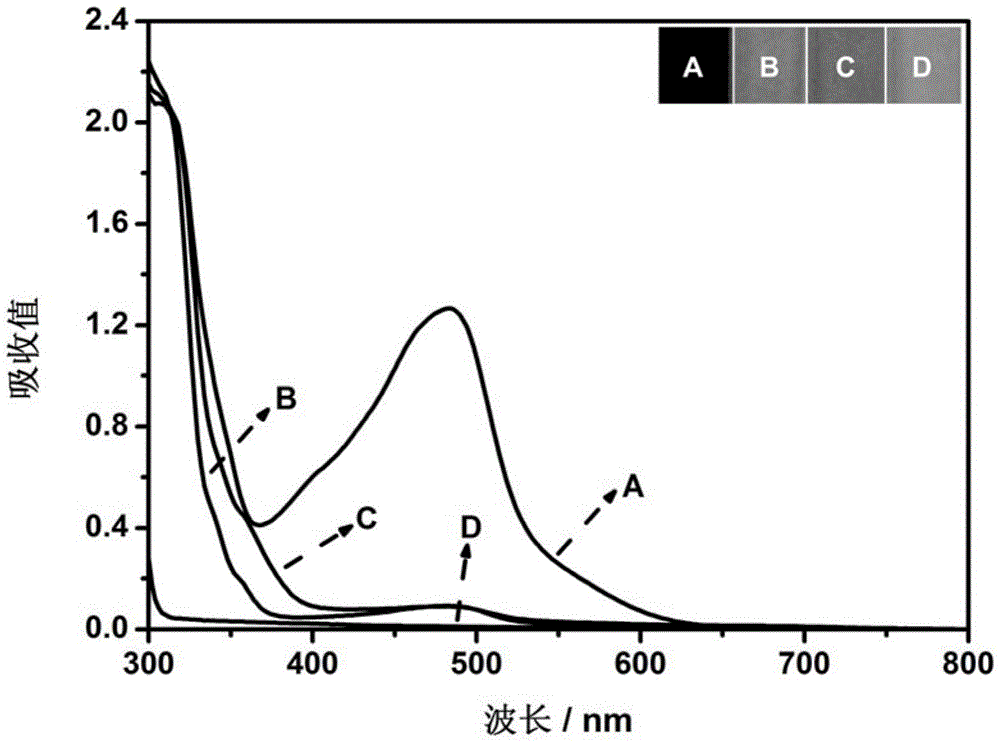
[0027] 20 μL acid phosphatase solution (100mU·mL -1 ) with 20 μL-ascorbic acid-2-phosphate (0.3M), 10 μL copper sulfate-Yulketonin disulfonate complex (copper sulfate: 12 mM, yloketone sodium disulfonate: 24 mM) and 550 μL glycine-HCl buffer (50mM, pH5.7) were evenly mixed, and the resulting mixture was incubated at 25°C for 15min, then the UV-Vis absorption spectrum of the solution in the range of 800-300nm was measured and the color change of the solution was recorded with a digital camera, as figure 2 shown. Among them, A means that all reagents are added, B means that acid phosphatase is not added, C means that L-ascorbic acid-2-phosphate is not added, and D means that copper sulfate-bathone sodium disulfonate complex is not added. The reagents were replaced with an equal amount of 50 mM glycine-hydrochloric acid buffer at pH 5.7. From ...
Embodiment 2
[0028] Example 2: The relationship between the ultraviolet-visible absorption spectrum and color change of the detection solution and the activity of acid phosphatase.
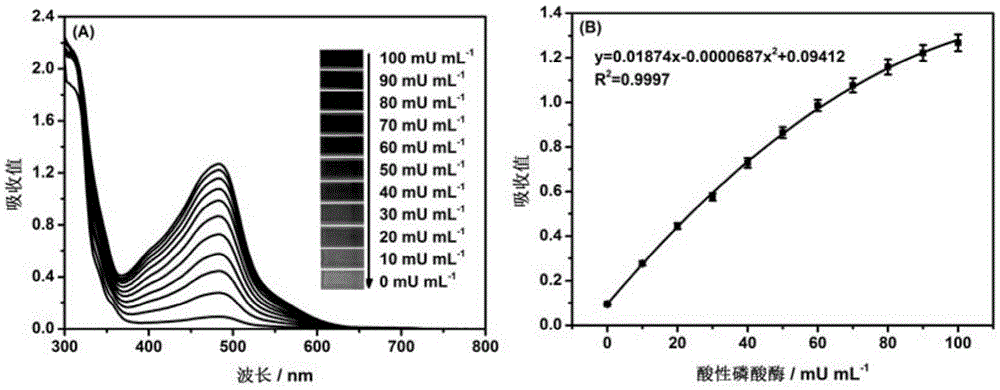
[0029] 20 μL of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 mU·mL -1 The acid phosphatase solution was mixed with 20 μL-ascorbic acid-2-phosphate (0.3M), 10 μL copper sulfate-thyroxine sodium disulfonate complex (copper sulfate: 12 mM, yloxone disulfonate sodium: 24 mM) and 550 μL of glycine-hydrochloric acid buffer solution (50 mM, pH5.7) was evenly mixed, and the resulting mixture was incubated at 25°C for 15 min, and then the UV-visible absorption spectrum of the solution in the range of 800-300 nm was measured and recorded with a digital camera The color changes, such as image 3 shown. From image 3 (A) It can be seen that with the increase of acid phosphatase activity in the sample, the absorption value of the detection solution at 484nm increases correspondingly. From image 3 (B) It can be seen ...
Embodiment 3
[0030] Example 3: Selective analysis of the colorimetric analysis of acid phosphatase activity by the method of the present invention.
[0031]20μL 1mg·mL -1 Acid Phosphatase (A), Bovine Serum Albumin (B), Hemoglobin (C), Horseradish Peroxidase (D), Streptavidin (E), Thrombin (F), Trypsin (G) Or 50mM pH5.7 glycine-hydrochloric acid buffer (H) was mixed with 20μL-ascorbic acid-2-phosphate (0.3M), 10μL copper sulfate-thyroxine sodium disulfonate complex (copper sulfate: 12mM, yloxone Sodium disulfonate: 24mM) and 550μL of glycine-hydrochloric acid buffer (50mM, pH5.7) were uniformly mixed, and the resulting mixture was incubated at 25°C for 15min, then the absorbance of the solution at 484nm was measured and recorded with a digital camera Record the color change of the solution as Figure 4 shown. It can be seen from the figure that except acid phosphatase, other interfering proteins will not cause obvious changes in the absorption value or color of the detection solution at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com