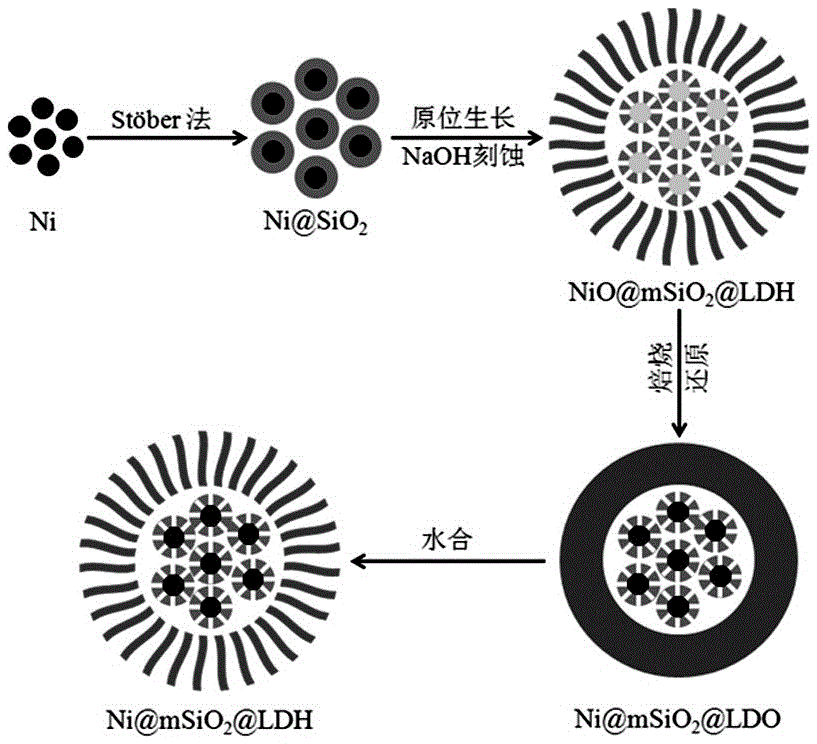
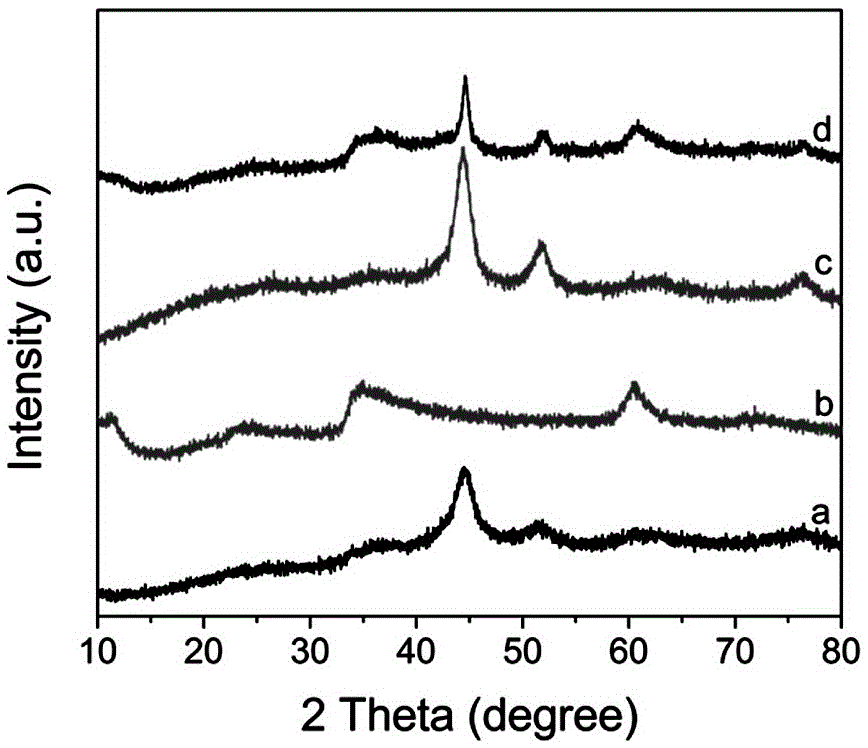
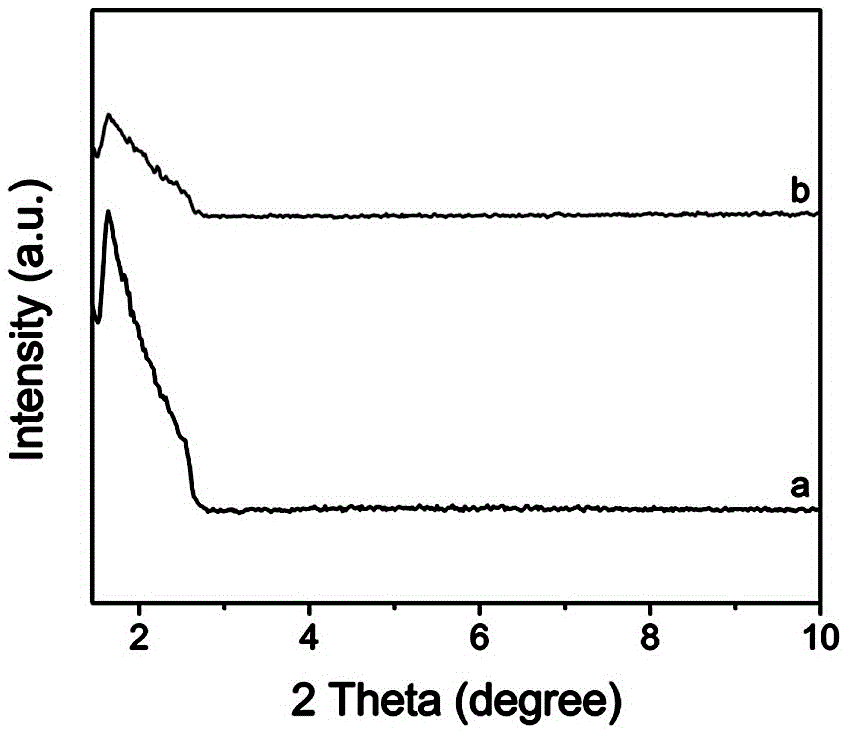
Nickel-based core-shell structured nano catalysis material and preparation method and application thereof
A technology of nano-catalytic materials and core-shell structure, which is applied in the field of catalytic materials and its preparation, can solve the problems of low hydrogenation selectivity, difficulty in popularization and application, and high price, and achieve good dispersion, simple and rapid preparation method, and low manufacturing cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) 1.7gNi(CH 3 COO) 2 4H 2 O was dissolved in 200mL of ethylene glycol, 2.24g of polyvinylpyrrolidone (PVP) was added, and stirred until the PVP was completely dissolved; then, under reflux, the solution was heated to 150°C, and 1.9g of NaBH was added at this temperature 4 , then the solution turned black, and continued heating and stirring for 2h, then stopped heating and stirring. After it is cooled, a small amount of acetone is added, centrifuged to obtain a black solid, washed with acetone, and dried at room temperature to obtain Ni nanoparticles;
[0039] (2) Take 0.4g of prepared Ni nanoparticles and add them to the mixed solution of ethanol (320mL) and water (80mL), ultrasonically disperse Ni for 30 minutes, then add 5.0mL ammonia solution (25wt%), and then slowly drop Add 1.0mL tetraethyl orthosilicate (TEOS), stir at room temperature for 5h, then use a magnet to separate, then wash with ethanol and water, and dry in an oven at 60°C for 6h to obtain NiSiO 2...
Embodiment 2
[0047] (1) 1.5gNi(CH 3 COO) 2 4H 2 O was dissolved in 200mL of ethanol, 2g of polyvinylpyrrolidone (PVP) was added, and stirred until the PVP was completely dissolved; then, under reflux, the solution was heated to 145°C, and 1.5g of NaBH was added at this temperature 4 , then the solution turns black, continue heating and stirring for 2h, then stop heating and stirring; after it cools down, add a small amount of acetone, centrifuge to obtain a black solid, wash with acetone, and dry at room temperature to obtain Ni nanoparticles;
[0048] (2) Add 0.4g of the prepared Ni nanoparticles into a mixed solution of ethanol (320mL) and water (80mL), ultrasonically disperse Ni for 30 minutes, then add 4.0mL of ammonia solution (30wt%), and then slowly drop Add 0.5mL tetraethyl orthosilicate (TEOS), stir at room temperature for 4.5h, then use a magnet to separate, then wash with ethanol and water, and dry in an oven at 60°C for 6h to obtain NiSiO 2 ;
[0049] (3) Aluminum isopropox...
Embodiment 3
[0055] (1) 2gNi(CH 3 COO) 2 4H 2 O was dissolved in 200mL of 1,5-pentanediol, 2.5g of polyvinylpyrrolidone (PVP) was added, and stirred until the PVP was completely dissolved; then, under reflux, the solution was heated to 155°C, and 2.5g of NaBH was added at this temperature 4 , then the solution turns black, continue heating and stirring for 2h, then stop heating and stirring; after it cools down, add a small amount of acetone, centrifuge to obtain a black solid, wash with acetone, and dry at room temperature to obtain Ni nanoparticles;
[0056] (2) Add 0.4g of the prepared Ni nanoparticles into a mixed solution of ethanol (320mL) and water (80mL), ultrasonically disperse Ni for 30 minutes, then add 6.0mL of ammonia solution (20wt%), and then slowly drop Add 1.5mL tetraethyl orthosilicate (TEOS), stir at room temperature for 5.5h, then use a magnet to separate, then wash with ethanol and water, and dry in an oven at 60°C for 6h to obtain NiSiO2;
[0057] (3) Dissolve alum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com