Preparation method of DNA base pair cross-linked supermolecular hydrogel
A technology of supramolecular hydrogel and base pairing, applied in the field of polymer hydrogel, can solve problems such as unstable structure, slow response, and inability to maintain shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
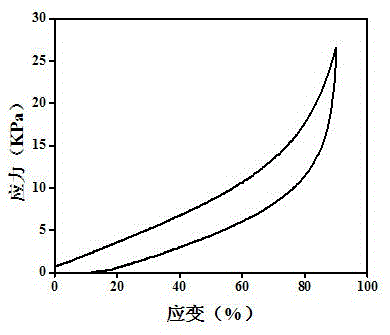
Embodiment 1
[0029] (1) Modification of bases
[0030] a. Dissolve 0.0031mol (0.6g) adenine in 20mL dimethylformamide (DMF) to prepare an adenine solution with a molar concentration of 0.15mol / L; dissolve 0.0031mol (0.56g) thymine in 20mL DMF , and prepare a thymine solution with a molar concentration of 0.15 mol / L; then add 0.0043 mol triethylamine to each of the above solutions, stir in an ice bath for 20 minutes, add 0.0034 mol acryloyl chloride, and pump the system into Vacuum, stirred and reacted at room temperature for 6 hours, respectively to obtain solutions of modified adenine base monomers and thymine base monomers; the molar ratios of adenine and thymine to acryloyl chloride and triethylamine were both 1:1.1 :1.4;
[0031] b. The modified adenine base monomer and thymine base monomer in the solution obtained in step a are precipitated with 140mL ether solvent respectively, and the ratio of ether solvent to the volume of the settled solution is 7:1, and then filter through a fu...
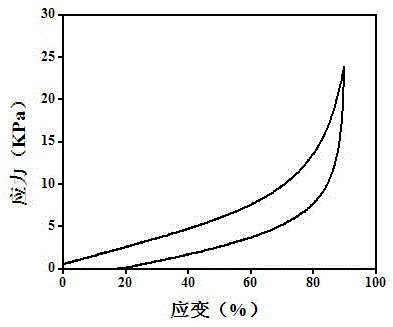
Embodiment 2
[0035] (1) Modification of bases
[0036] a. Dissolve 0.0040mol (0.75mL) adenine in 20mL dimethylformamide (DMF) to prepare an adenine solution with a molar concentration of 0.20mol / L; dissolve 0.0040mol (0.72g) thymine in 20mL DMF , and prepare a thymine solution with a molar concentration of 0.20 mol / L; then add 0.0056 mol triethylamine to each of the above solutions, stir in an ice bath for 25 minutes, add 0.0044 mol acryloyl chloride, and pump the system into Vacuum, stirred and reacted at room temperature for 7 hours, respectively to obtain solutions of modified adenine base monomers and thymine base monomers; the molar ratios of adenine and thymine to acryloyl chloride and triethylamine were both 1:1.1 :1.4;
[0037] b. The modified adenine base monomer and thymine base monomer in the solution obtained in step a are precipitated with 160mL ether solvent respectively, and the ratio of ether solvent to the volume of the settled solution is 8:1, and then filter through a ...
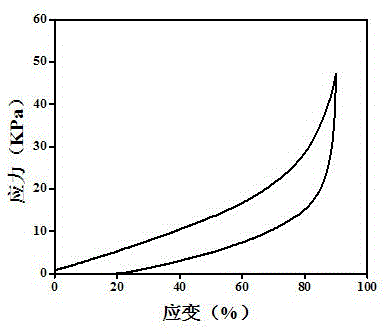
Embodiment 3
[0041] (1) Modification of bases
[0042] a. Dissolve 0.0050mol (0.94g) adenine in 20mL dimethylformamide (DMF) to prepare an adenine solution with a molar concentration of 0.25mol / L; dissolve 0.0050mol (0.90g) thymine in 20mL DMF , and prepare a thymine solution with a molar concentration of 0.25 mol / L; then add 0.0075 mol triethylamine to each of the above solutions, stir in an ice bath for 30 minutes, add 0.0060 mol acryloyl chloride, and pump the system into Vacuum, stirred and reacted at room temperature for 8 hours, respectively obtained solutions of modified adenine base monomer and thymine base monomer; the molar ratios of adenine and thymine to acryloyl chloride and triethylamine were both 1:1.2 :1.5;
[0043] b. The modified adenine base monomer and thymine base monomer in the solution obtained in step a are precipitated with 180mL ether solvent respectively, the ratio of ether solvent to the volume of the settled solution is 9:1, and then filter through a funnel t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com