Photo-responsive random copolymer and preparation method thereof
A random copolymer, photoresponsive technology, applied in the field of photoresponsive random copolymer and its preparation, to achieve the effect of simple preparation method, good polymerization controllability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
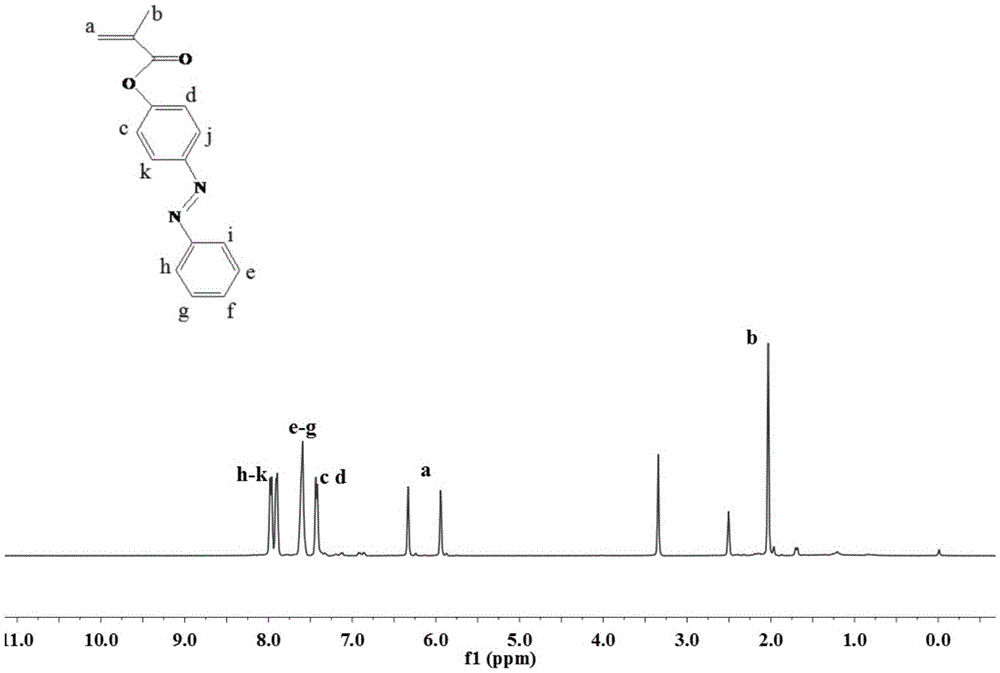
[0029] (1) Synthesis of AzoMA monomer: Add 4-hydroxyazobenzene (1.9823g, 10mmoL), THF (50mL) and TEA (1.67mL, 12mmoL) into a 250mL round bottom flask equipped with a dropping funnel and a magnetic stir bar ), and magnetically stirred evenly to 0 °C under the condition of ice bath. Continue stirring in the ice bath, and slowly drop methacryloyl chloride (1.94 mL, 20 mmoL) dissolved in THF (40 mL) into the above mixed solution with a dropping funnel. React at room temperature for 15 hours, remove the generated triethylamine hydrochloride solid by filtration, collect the filtrate for rotary evaporation, dissolve the obtained solid in dichloromethane and place it in a separatory funnel, wash it with distilled water for 3 times, then add 5 g of anhydrous sulfuric acid Magnesium was dried and filtered, and the resultant dichloromethane solution was obtained by filtration. After the dichloromethane was removed by rotary evaporation, the crude product was obtained. The crude product w...
Embodiment 2
[0032] (1) Step (1) is the same as in Example 1.
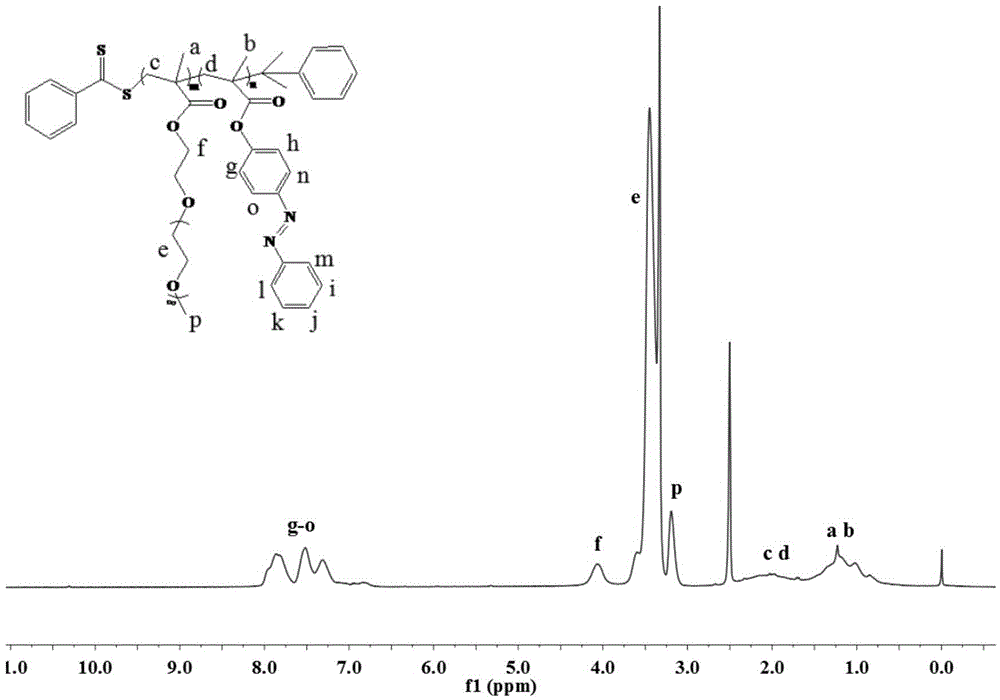
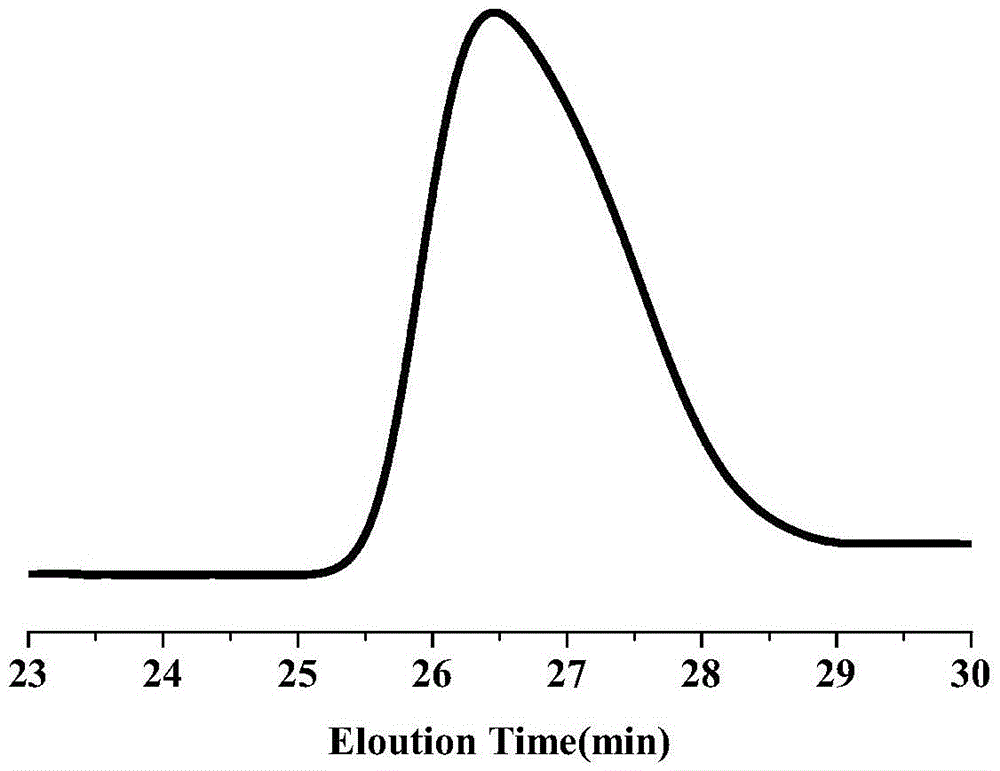
[0033] (2) Synthesis of P(PEGMA-co-AzoMA): PEGMA (0.25g, 0.5mmoL), AzoMA (0.266g, 1mmoL), CDB (0.01361g, 0.05mmoL), AIBN (0.00164g, 0.01mmoL) and Add THF (2 mL) into a 25 mL Shlenk tube, freeze and thaw continuously for 3 to 5 times, then fill with argon protection, polymerize at 75°C for 40 h, and quench with liquid nitrogen to end the reaction. The reaction mixture was precipitated in 50 mL of n-hexane, washed and purified three times, filtered to remove unreacted AzoMA, the obtained crude product was dissolved in 2 mL of THF, and dialyzed in distilled water for 48 hours to remove unreacted PEGMA, and finally dark red viscous The yield of liquid P(PEGMA-co-AzoMA) is 63%, the values of m and n are 4.0 and 8.0 respectively, and the average molecular weight is 4112.
Embodiment 3
[0035] (1) Step (1) is the same as in Example 1.
[0036](2) Synthesis of P(PEGMA-co-AzoMA): PEGMA (0.1g, 0.2mmoL), AzoMA (0.266g, 1mmoL), CDB (0.01361g, 0.05mmoL), AIBN (0.00164g, 0.01mmoL) and Add THF (2 mL) into a 25 mL Shlenk tube, freeze and thaw continuously for 3 to 5 times, and then fill with argon for protection. Polymerize at 75°C for 36 h, and quench with liquid nitrogen to end the reaction. The reaction mixture was precipitated in 50 mL of n-hexane, washed and purified three times, filtered to remove unreacted AzoMA, the obtained crude product was dissolved in 2 mL of THF, dialyzed in distilled water for 48 hours to remove unreacted PEGMA, and finally dark red viscous The yield of liquid P(PEGMA-co-AzoMA) is 60%, the values of m and n are 1.6 and 8.0 respectively, and the average molecular weight is 2921.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com