Synthesis of ciprofloxacin propionate antigen
A technology of ciprofloxacin and propionic acid, which is applied in the field of ciprofloxacin hapten and its preparation method and application, and can solve problems such as drug residues and threats to human health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the preparation of ciprofloxacin propionate
[0046] Take ciprofloxacin hydrochloride 0.33g (1mmol), dissolve it in 10ml of DMF solution, stir to dissolve at 80°C, add 0.18g (1.2mmol) of 3-bromopropionic acid hydrobromide dropwise, stir at 80°C for 3h, after the reaction The solvent is evaporated off from the reaction solution under reduced pressure to obtain the crude product of ciprofloxacin propionate shown in formula (I). By preparing the liquid phase (Column: Sunfire, (3.5um, 150*4.6mm) mobile phase A: H 2 O(0.01%TFA)‐B: Acetonitrile (0.01%TFA) 1‐9min 5%B—95%B, 9‐14min, 95%B, 14‐15min95%B—5%B, flow rate 1.0mL / min, column temperature 40°C) to obtain pure ciprofloxacin propionate.
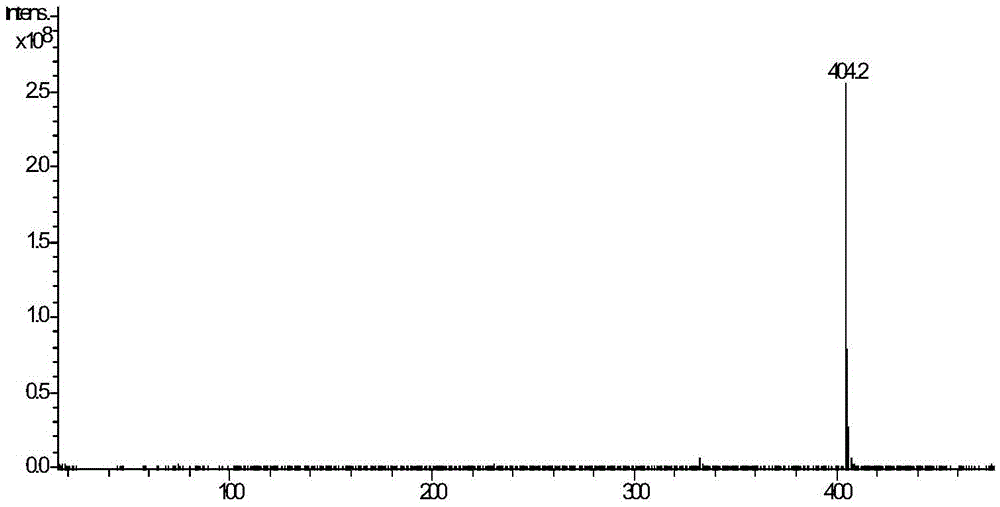
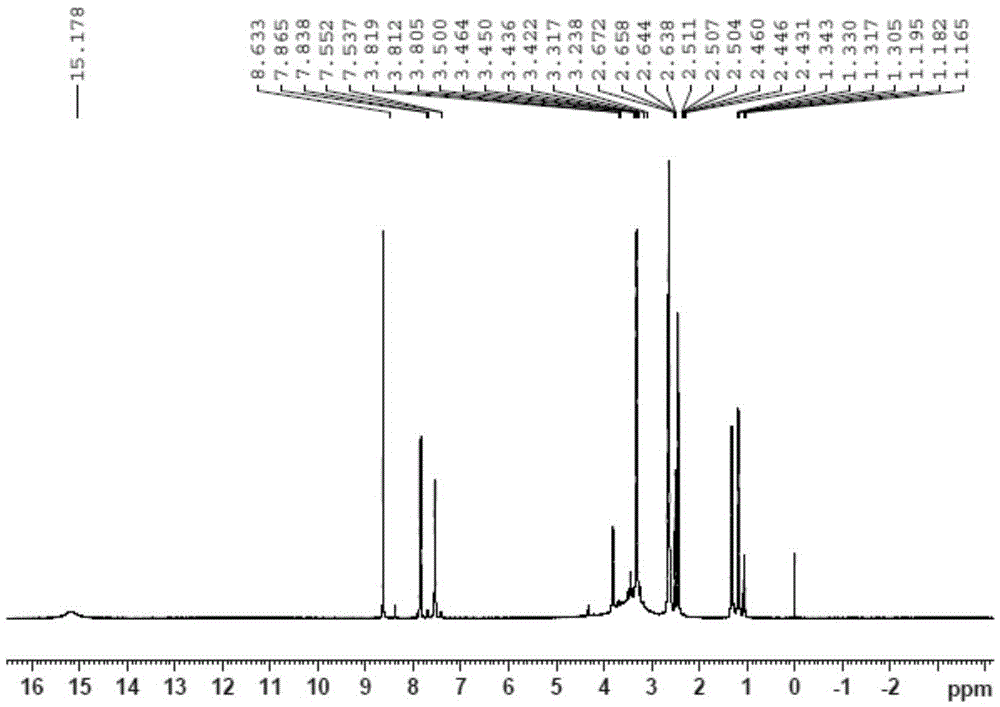
[0047] It was identified by infrared spectroscopic scanner, ESI‐MS and nuclear magnetic resonance.
[0048]
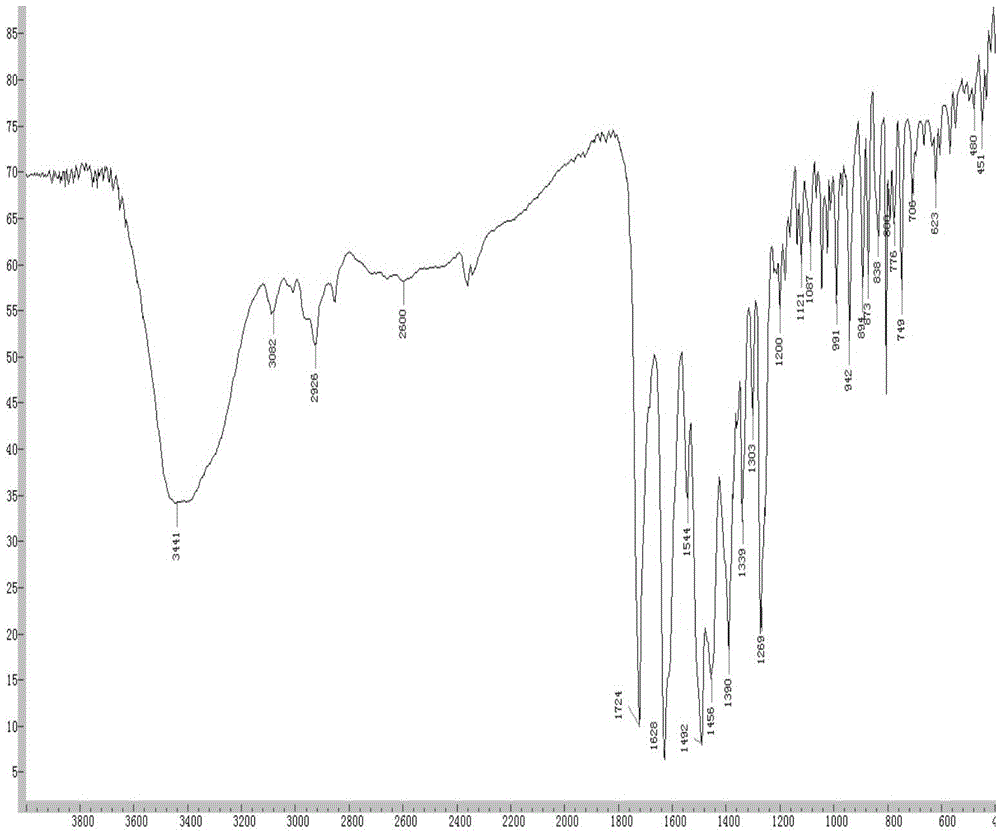
[0049] The infrared spectrum of formula (I) ciprofloxacin propionate shows that wavenumber 3373cm ‐1 The broad absorption peak is the absorption peak of the c...
Embodiment 2
[0053] Embodiment 2: Synthesis of ciprofloxacin-propionic acid-bovine serum albumin (CIP-C) by glutaraldehyde method 2 h 4 -COO-BSA)
[0054] Weigh 0.10 g of ciprofloxacin propionate prepared in Example 1 with an analytical balance, dissolve it in 5 mL of 0.6 mol / L HCl solution, add 30 mg of zinc powder, and heat in a water bath at 80° C. for 30 min to obtain solution A. Weigh 100 mg bovine serum albumin (BSA) and dissolve it in 2 mL of 0.02 mol / L PBS solution to obtain solution B. Slowly add solution A to solution B dropwise and mix well, add 0.1ml of 25% glutaraldehyde dropwise, and stir at room temperature for 6h. Put the mixed solution into a dialysis bag and dialyze with 0.01mol / L PBS solution (pH7.4) for 72 hours at 4°C to obtain ciprofloxacin-propionic acid-bovine serum albumin CIP-C 2 h 4 -COO-BSA. Store at -20°C.
[0055] Simultaneously to the formula (I) ciprofloxacin propionate (CPLX‐C) prepared by embodiment 1 2 h 4 ‐COOH), ciprofloxacin‐propionic acid‐bovi...
Embodiment 3
[0057] Embodiment 3 carbodiimide method synthesis ciprofloxacin-propionic acid-ovalbumin (CPLX-C 2 h 4 -COO-OVA)
[0058] Get ciprofloxacin propionate 0.10g prepared in Example 1, dissolve in N,N-dimethylformamide (DMF), obtain solution C, take 1-(3-dimethylaminopropyl)-3- Ethylcarbodiimide hydrochloride (EDC) 30 mg was fully dissolved in 4 mL of distilled water, slowly added dropwise to solution C, and stirred at room temperature for 24 hours to obtain solution D. After centrifugation, the supernatant was slowly added dropwise to 5 mL of sodium carbonate buffer solution (0.1 mol / L) containing 50 mg ovalbumin (OVA). Stir in a refrigerator at 4°C for 72 hours to obtain the ciprofloxacin-propionic acid-ovalbumin antigen (CIP-C 3 h 7 -NH 2 ‐OVA)), stored at ‐20°C.
[0059] Simultaneously to the formula (I) ciprofloxacin propionate (CPLX‐C) prepared by embodiment 1 2 h 4 -COOH), ciprofloxacin-propionic acid-ovalbumin (CPLX-C) prepared in embodiment 3 2 h 4 ‐COO‐OVA), ova...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com