Stable nystatin medicine composition and preparation method thereof
A technology of nystatin and composition, applied in the field of nystatin pharmaceutical composition and preparation thereof, can solve the problems of no therapeutic effect of systemic fungal infection, unsatisfactory curative effect, low blood drug concentration, etc., and achieve optimal suspension stability, Improved convenience and compliance, easy viscosity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
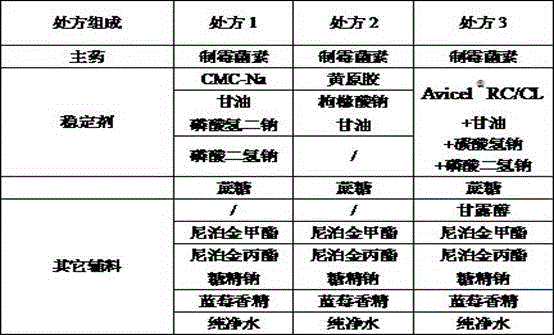
[0038] Embodiment 1 Nystatin Oral Suspension
[0039] Drug-stabilizer complex:
[0040] Nystatin 8.8g
[0041] 2g
[0042] Sodium bicarbonate 5g
[0043] Sodium dihydrogen phosphate 10g
[0044] Purified water 50g
[0045] Glycerin 60ml
[0046] Sucrose 50g
[0047] Suspension additives:
[0048] Sucrose 200g
[0049] Methylparaben 1g
[0050] Blueberry essence 1ml
[0051] Appropriate amount of water
[0052] Makes 500ml
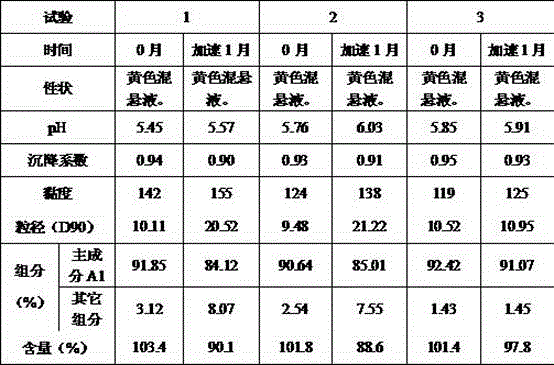
[0053] The raw material of nystatin is micronized so that its particle size ranges from 8 to 12 μm; , sodium bicarbonate, and sodium dihydrogen phosphate were dispersed in about 100ml of water, added glycerin, and stirred at 15,000rpm for 3 minutes to prepare a combined stabilizer; put the above combined stabilizer in a high-pressure homogenizer, and add the prescribed amount The micronized raw material of nystatin is circulated three times at 100-200 bar, and five times at 300-500 bar, so that the drug is completely coated by the combined s...
Embodiment 2
[0054] Embodiment 2 Nystatin oral dry suspension
[0055] Drug-stabilizer complex:
[0056] Nystatin 8.8g
[0057] 2.0g
[0058] Polysorbate - 8060ml
[0059] Sodium bicarbonate 4.0g
[0060] Sodium dihydrogen phosphate 6.5g
[0061] Methylparaben 0.5g
[0062] Sodium saccharin 0.05g
[0063] Sucrose 30.0g
[0064] 40g of water
[0065] Dry suspension additives:
[0066] Mannitol 180g
[0067] Lactose 200g
[0068] Appropriate amount of ethanol water
[0069] Made into 500g
[0070] The nystatin raw material is micronized so that the particle size ranges from 15 to 20 μm; the sucrose is pulverized and passed through a 100-mesh sieve. Will , sodium bicarbonate, and sodium dihydrogen phosphate were dispersed in about 20ml of water, polysorbate-80 was added, and stirred at 10,000rpm for 5min to prepare a combined stabilizer. Put the above combined stabilizer in a high-pressure homogenizer, add the prescribed amount of micronized nystatin raw material at the same...
Embodiment 3
[0071] Embodiment 3 Nystatin Granules
[0072] Drug-stabilizer complex:
[0073] Nystatin 9.0g
[0074] 2.5g
[0075] Sodium bicarbonate 3.0g
[0076] Glycerin 50ml
[0077] Citric acid 8.0g
[0078] Purified water 30.0g
[0079] Granules and other additives:
[0080] Lactose 250.0g
[0081] Mannitol 100.0g
[0082] Sucrose 100.0g
[0083] Methylparaben 0.5g
[0084] Propylparaben 0.1g
[0085] Sodium saccharin 0.05g
[0086] Made into 500g
[0087] The nystatin raw material is micronized so that the particle size ranges from 10 to 15 μm. Will , sodium bicarbonate, sodium citrate, and citric acid were dispersed in about 15ml of water, added glycerin, and stirred at 10,000~20,000rpm for 3 minutes to prepare a combined stabilizer. Put the above combined stabilizer in a high-pressure homogenizer, add the prescribed amount of micronized nystatin raw material at the same time, cycle 2 times at 200 bar, and cycle 4 times at 400 bar, so that the drug is completely w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com