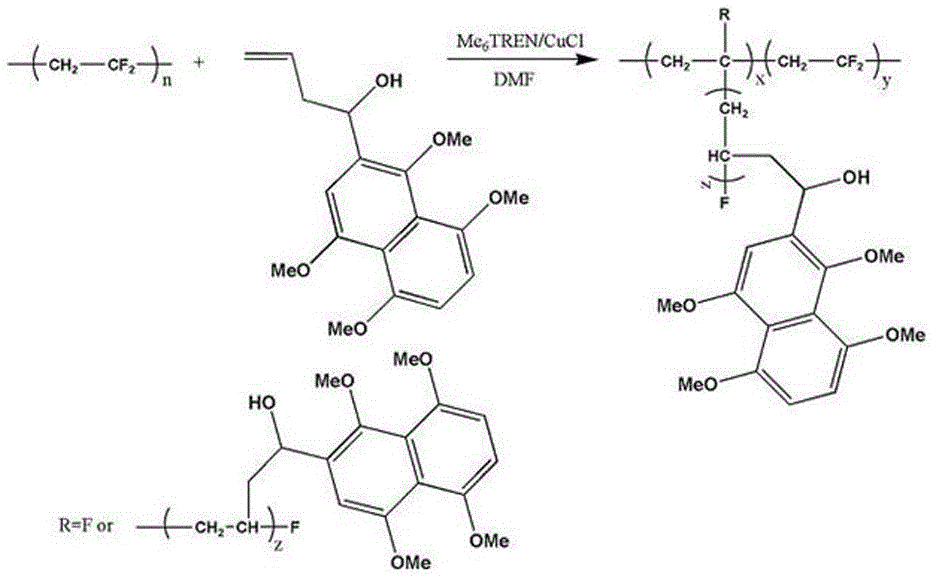
Preparation method of anthraquinone functionalized polyvinylidene fluoride ultrafiltration membrane
A polyvinylidene fluoride and functional technology, which is applied in chemical instruments and methods, ultrafiltration, membranes, etc., can solve problems such as secondary pollution of water bodies, shedding and loss of quinones, and achieve the effect of promoting degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
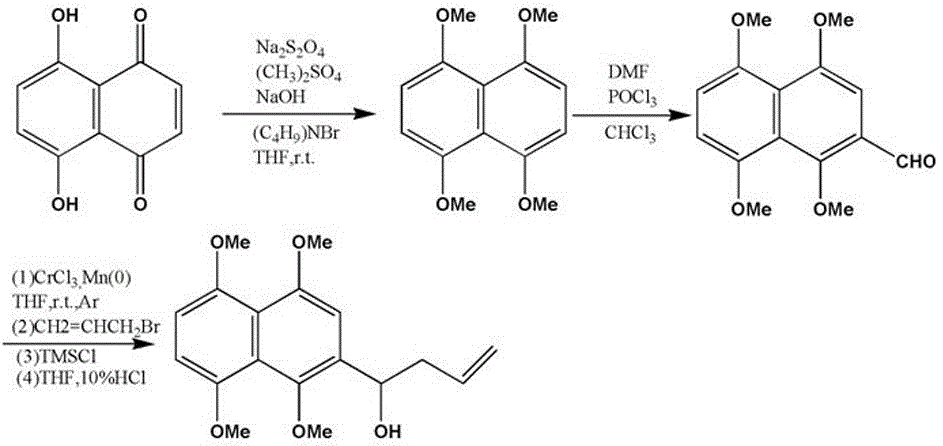
[0035] A preparation method of anthraquinone functionalized polyvinylidene fluoride ultrafiltration membrane, comprising the following steps:
[0036] (1) Synthesis of 2-(1-hydroxy-3-butene)-1,4,5,8-tetramethoxynaphthalene:
[0037] ①Synthesis of 1,4,5,8-tetramethoxynaphthalene: Add naphthalene, catalytic amount of tetrabutylammonium bromide, and tetrahydrofuran into a round-bottomed flask, stir until dissolved, then add aqueous sodium dithionite and disulfite Methyl ester solution, stir until the solution is uniform; move the round-bottomed flask to an ice-water bath, react for 1 h, slowly add NaOH aqueous solution dropwise, remove the ice-water bath after the dropwise addition, react at room temperature for 30 min, stir at a constant speed for 18 h until the reaction is complete, The reaction solution was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, recovered ethyl acetate under reduced pressure, and separated b...
Embodiment 2
[0048] A preparation method of anthraquinone functionalized polyvinylidene fluoride ultrafiltration membrane, comprising the following steps:
[0049] (1) Synthesis of 2-(1-hydroxy-3-butene)-1,4,5,8-tetramethoxynaphthalene:
[0050] ①Synthesis of 1,4,5,8-tetramethoxynaphthalene: Add naphthalene, catalytic amount of tetrabutylammonium bromide, and tetrahydrofuran into a round-bottomed flask, stir until dissolved, then add aqueous sodium dithionite and disulfite Methyl ester solution, stir until the solution is uniform; move the round-bottomed flask to an ice-water bath, react for 1 h, slowly add NaOH aqueous solution dropwise, remove the ice-water bath after the dropwise addition, react at room temperature for 30 min, stir at a constant speed for 18 h until the reaction is complete, The reaction solution was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, recovered ethyl acetate under reduced pressure, and separated b...
Embodiment 3
[0060] A preparation method of anthraquinone functionalized polyvinylidene fluoride ultrafiltration membrane, comprising the following steps:
[0061] (1) Synthesis of 2-(1-hydroxy-3-butene)-1,4,5,8-tetramethoxynaphthalene:
[0062]①Synthesis of 1,4,5,8-tetramethoxynaphthalene: Add naphthalene, catalytic amount of tetrabutylammonium bromide, and tetrahydrofuran into a round-bottomed flask, stir until dissolved, then add aqueous sodium dithionite and disulfite Methyl ester solution, stir until the solution is uniform; move the round-bottomed flask to an ice-water bath, react for 1 h, slowly add NaOH aqueous solution dropwise, remove the ice-water bath after the dropwise addition, react at room temperature for 30 min, stir at a constant speed for 18 h until the reaction is complete, The reaction solution was extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, recovered ethyl acetate under reduced pressure, and separated by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com