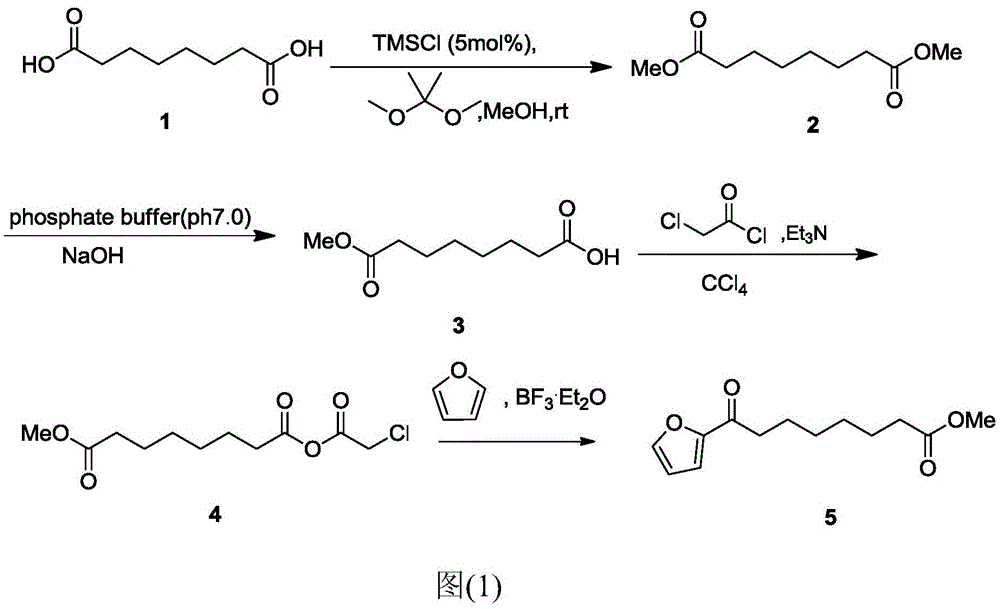
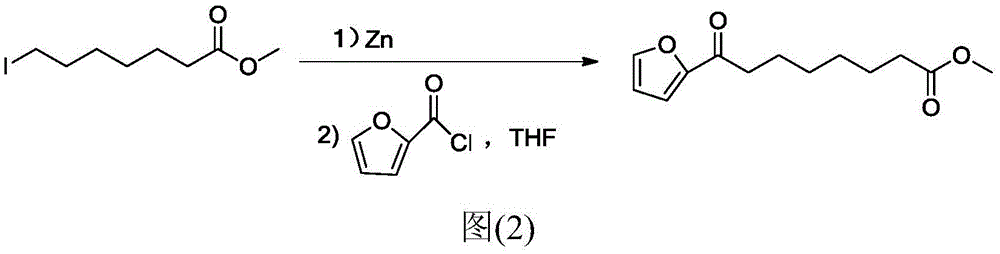
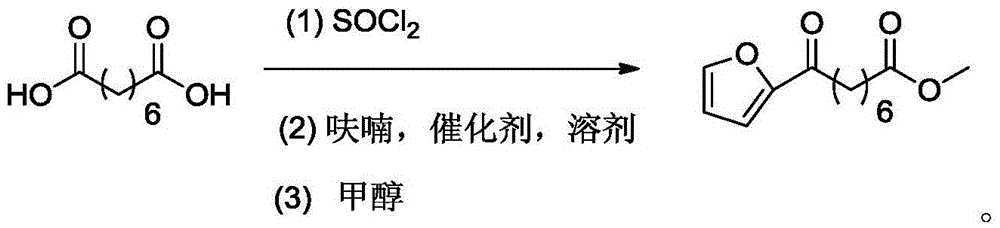
Chemical synthetic method of 8-furan-8-oxomethyl caprylate
A technology for methyl octanoate and chemical synthesis, which is applied in the field of methyl 8-furan-8-oxoctoate, and can solve the problems of limited industrial application, complicated anhydrous and oxygen-free operations, and highly toxic cuprous cyanide. , to achieve the effect of low cost, shortened reaction time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add suberic acid (5.0g, 0.029mol) and thionyl chloride (4.4ml, 0.061mol) into a 50mL two-necked bottle equipped with a thermometer, connect the tail gas absorption device, and the absorption solution is a saturated sodium hydroxide solution at room temperature Down reaction 12 hours, stop reaction, remove superfluous thionyl chloride by normal pressure distillation and obtain colorless liquid suberoyl chloride, add 10ml nitromethane successively in bottle, boron trifluoride ether (1.8ml, 0.014mol), in Furan (2.8ml, 0.038mol) was added dropwise at 35°C, and the addition was completed in about 5 minutes. After continuing the reaction for 10 minutes, the reaction flask was placed in an ice-water bath at 0°C and 1.5ml of methanol was added dropwise to quench the reaction. After the solvent was distilled off under reduced pressure, 30ml of mixed solvent (petroleum ether / ethyl acetate=10:1) was added to extract the target product, filtered and spin-dried to obtain a crude yell...
Embodiment 2
[0028] Add suberic acid (5.0g, 0.029mol) and thionyl chloride (4.8ml, 0.066mol) in the 50ml two-necked bottle equipped with a thermometer, connect the tail gas absorption device, the absorption liquid is saturated sodium hydroxide solution, room temperature The reaction was stopped for 12 hours, and the excess thionyl chloride was removed by normal pressure distillation to obtain a colorless liquid suberoyl chloride. In the bottle, 15ml of n-heptane, boron trifluoride ether (1.16ml, 0.009mol), 50 At ℃, furan (2.9ml, 0.004mol) was added dropwise to the system with a constant pressure dropping funnel, and the dropwise addition was completed in about 5 minutes. After continuing the reaction for 20 minutes, the reaction bottle was placed in an ice-water bath and 1.5ml of methanol was added dropwise to quench extinction reaction. After removing the solvent by distillation under reduced pressure, add 30ml of a mixed solvent (n-hexane / ethyl acetate=20:1) to extract the target product...
Embodiment 3
[0030] Add suberic acid (5.0g, 0.029mol) and thionyl chloride (4.4ml, 0.061mol) in a 50ml two-necked bottle equipped with a thermometer, connect the tail gas absorption device, the absorption liquid is a saturated sodium hydroxide solution, and The reaction was carried out at low temperature for 12 hours, the reaction was stopped, and excess thionyl chloride was removed by atmospheric distillation to obtain a colorless liquid suberoyl chloride. Add 20ml of nitromethane and boron trifluoride diethyl ether (1.8ml, 0.014mol) to the bottle in turn, add furan (2.5ml, 0.034mol) dropwise to the system with a constant pressure dropping funnel at 50°C, drop for about 5 minutes After the addition is complete, continue the reaction for 10 minutes, place the reaction bottle in an ice-water bath and add dropwise 1.5ml of methanol to quench the reaction. After removing the solvent by distillation under reduced pressure, add 30ml of mixed solvent (petroleum ether / ethyl acetate=15:1) After ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com