Positive electrode for alkaline water electrolysis
A technology of water electrolysis and alkalinity, applied in the direction of electrodes, electrolysis components, electrolysis process, etc., can solve the problems of undisclosed stability, undisclosed manufacturing conditions of lithium content ratio, etc., and achieve the effect of maintaining corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
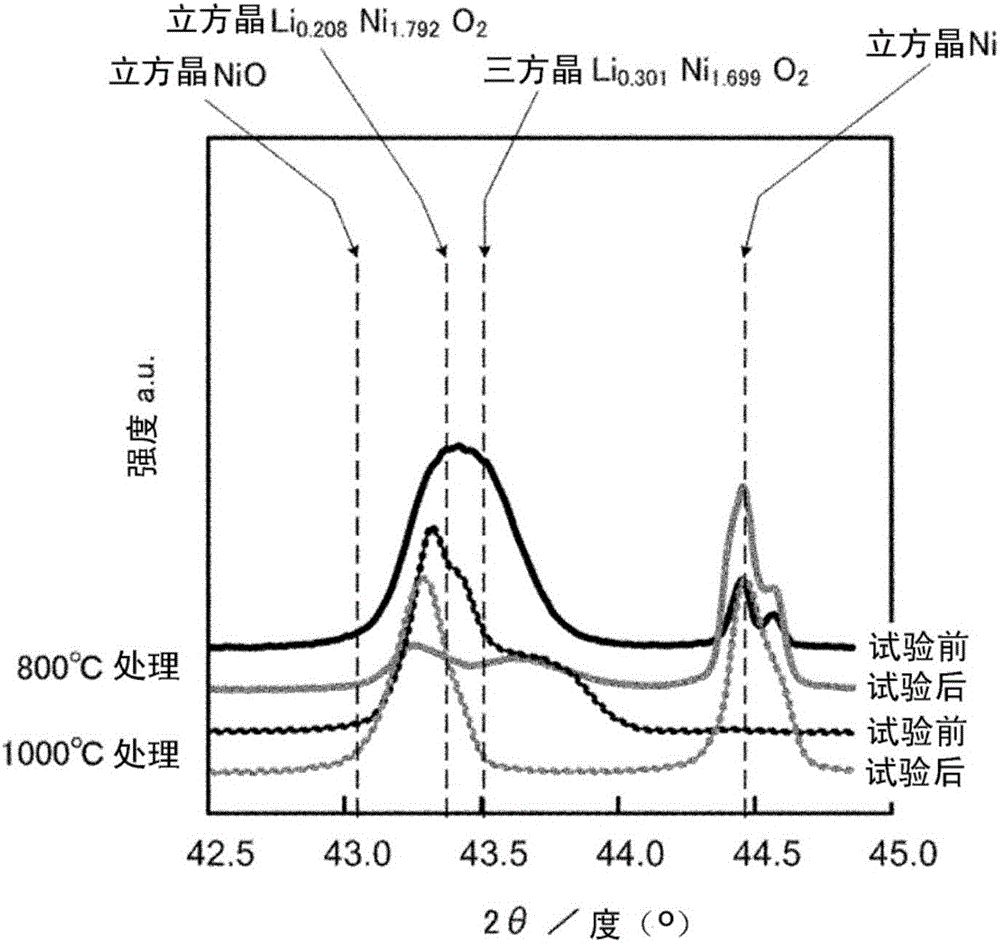
[0079] As the electrode substrate, a mirror-polished (P8000, 1-micron particle size) nickel plate at room temperature was used, immersed in 5 mass % lithium hydroxide for 1 hour, and calcined in an air atmosphere of 1000° C. for 1 hour to prepare a lithium oxide electrode with nickel oxide. As the electrode of the obtained lithium nickel oxide from image 3 X-ray presumption of , Li 0.208 Ni 1.792 O 2 As the main component, the molar ratio of lithium to nickel (Li / Ni) was 0.12 on average. from image 3 It can be seen from the X-ray diffraction analysis of 0.208 Ni 1.792 O 2 The peaks are shown as sharp peaks.
[0080] The temperature of the following electrochemical measurement was set to 25±1° C., and it was measured in a 25 mass % potassium hydroxide aqueous solution. As an electrochemical pretreatment, in the potential range: 0 ~ 1.5Vvs.RHE, scanning speed 100mVs -1 Cyclic Voltammetry (CV) was performed for 100 cycles. Cycle test at potential 1.0~1.8Vvs.RHE, scan...
Embodiment 2
[0083] An electrode having a lithium oxide and a nickel oxide was produced in the same manner as in Example 1, except that it was fired in an air atmosphere at 900° C. for 1 hour as an oxidation treatment. The molar ratio of lithium to nickel (Li / Ni) of the obtained lithium nickel oxide electrode was 0.14.
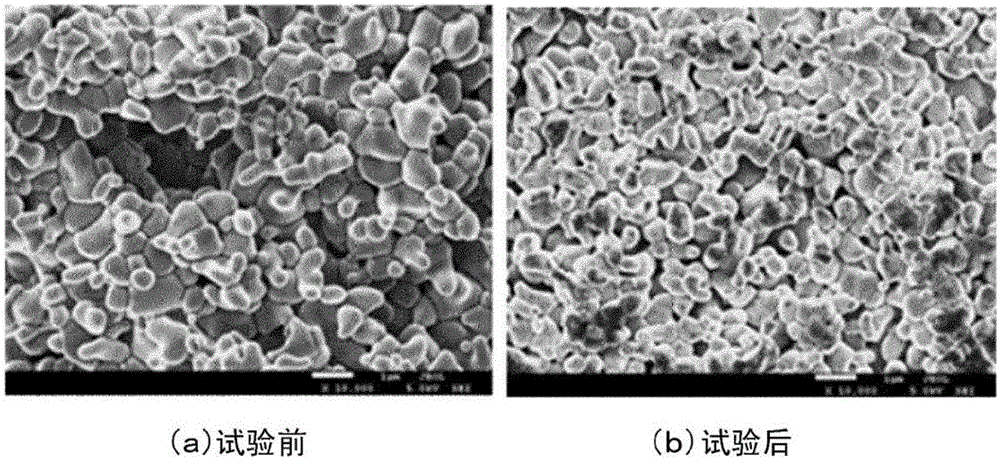
[0084] When performing the same cycle test, 100mAcm -2 The overpotential at the initial stage is 210mV, and the overpotential after the cycle test is 250mV, which is basically stable. No large crystal state change was recognized in the electron microscope photographs. In the X-ray diffraction analysis of the electrodes before and after the cycle test, the same changes as in Example 1 were confirmed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| voidage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com