Synthesis method of 2,3-dihydrobenzofuran
A synthesis method and technology of dihydrobenzene, applied in the direction of organic chemistry, etc., can solve the problems of many reaction by-products, reduced yield and product quality, and low yield, and achieve the goal of increasing yield, reducing energy consumption, and optimizing the reaction process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the catalyst is as follows: mixing cupric chloride and ferric chloride according to mass ratio, pressing into tablets, drying at 150-200 DEG C and calcining at 500-550 DEG C to obtain it.
[0034] The effect of the solid mass addition of copper chloride and ferric chloride mixed solid catalyst on the yield of 2-phenoxyethanol was tested, and the results are shown in Table 1.
[0035] Table 1: The effect of copper chloride and ferric chloride solid addition quality on the yield of 2-phenoxyethanol
[0036] Experiment number 1 2 3 4 5 6 7 8 9 Copper chloride (g) 30 0 15 22.5 25 26.3 7.5 5 26.3 Ferric chloride (g) 0 30 15 7.5 5 3.7 22.5 25 3.7 Yield(%) 80 78 79 92 90 82 85 84 83
[0037] When the mass ratio of copper chloride to ferric chloride is 3:1-5:1, the yield of 2-phenoxyethanol is significantly improved.
[0038] The solid mass ratio of copper chloride and ferric chloride was...
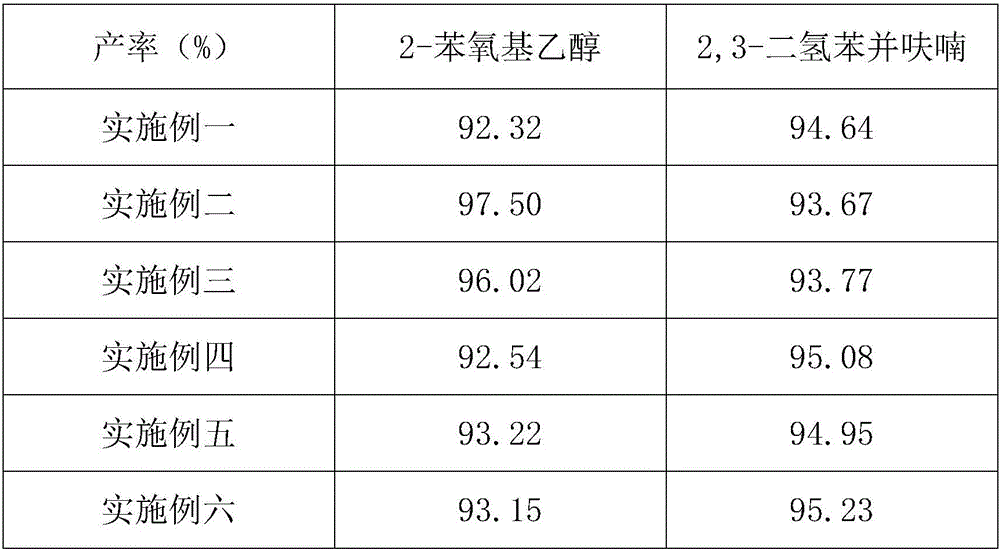
Embodiment 1
[0051] A kind of synthetic method of 2,3-dihydrobenzofuran, comprises the following steps:
[0052] The first step: Synthesis of 2-phenoxyethanol:
[0053] Add 100ml of sodium phenate aqueous solution 100ml, 2-chloroethanol 67.1ml, cupric chloride and ferric chloride mixed solid 17.05g (the mass ratio of cupric chloride and ferric chloride is 3:1) in the 500ml container, heat Start timed reflux reaction at 60°C for 2 hours, cool to room temperature after the reaction to collect the organic layer, wash the organic layer twice with 3% aqueous sodium hydroxide solution to obtain 2-phenoxyethanol;
[0054] The second step: Synthesis of 2,3-dihydrobenzofuran:
[0055] Add the 2-phenoxyethanol synthesized in the first step above, 1 g of zinc chloride solid, and 3 g of manganese dichloride solid in a 250 ml container, mix and stir and heat to 200 ° C to start timing and reflux reaction for 3 hours. After the timing is over, slowly Cool down to room temperature, add 3% sodium hydrox...
Embodiment 2
[0057] A kind of synthetic method of 2,3-dihydrobenzofuran, comprises the following steps:
[0058] The first step: Synthesis of 2-phenoxyethanol:
[0059] Add 100ml of sodium phenate aqueous solution 100ml, 2-chloroethanol 100.65ml, cupric chloride and ferric chloride mixed solid 34.10g (the mass ratio of cupric chloride and ferric chloride is 5:1) in the 500ml container, heating Start timed reflux reaction at 70°C for 3 hours, cool to room temperature after the reaction to collect the organic layer, wash the organic layer with 5% aqueous sodium hydroxide solution for 3 times to obtain 2-phenoxyethanol;
[0060] The second step: Synthesis of 2,3-dihydrobenzofuran:
[0061] Add the 2-phenoxyethanol synthesized in the first step above, 1g of zinc chloride solid, and 5g of manganese dichloride solid into a 250ml container, mix and stir and heat to 220°C to start timed reflux reaction for 4 hours. Cool down to room temperature, add 5% sodium hydroxide aqueous solution to wash 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com