Method for efficiently producing transpeptidase sortase A by using recombinant escherichia coli
A technology of recombinant Escherichia coli and transpeptidase, applied in the field of genetic engineering, can solve problems such as the influence of protein structure, and achieve the effect of efficient production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Recombinant E. coli fermentation produces SortaseA
[0024] Using the genome of S.aureus as a template, amplified with srt-F and srt-R (Table 1) primers to obtain the srt gene that removes the N-terminal signal peptide, and transforming it into E.coliJM109 by connecting with the T vector. Then it was subcloned into plasmid pET28a and transformed into E. coli expression host E.coliBL21(DE3).
[0025] With TB medium (peptone 12g / L, yeast powder 24g / L, glycerol 4g / L, KH 2 PO 4 23.1g / L, K 2 HPO 4 125.4g / L) as fermentation medium, 50mL of 500mL shake flask liquid, 2% inoculum, fermentation temperature 37°C, induction fermentation time 3h, inducer IPTG concentration 100mM / L, induction timing OD 600 = 0.6. The yield of SortaseA was 135.4mg / L, and the enzyme activity per unit mass of bacteria was 692.1U / mg.
Embodiment 2
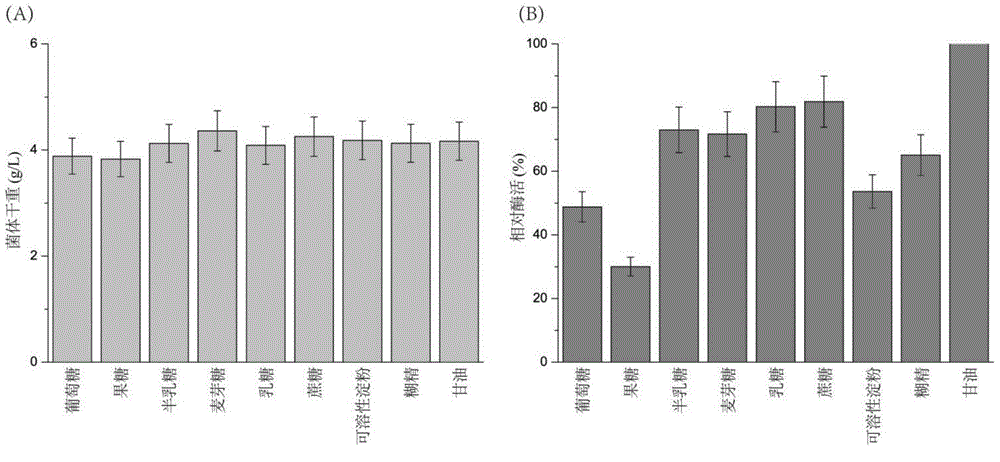
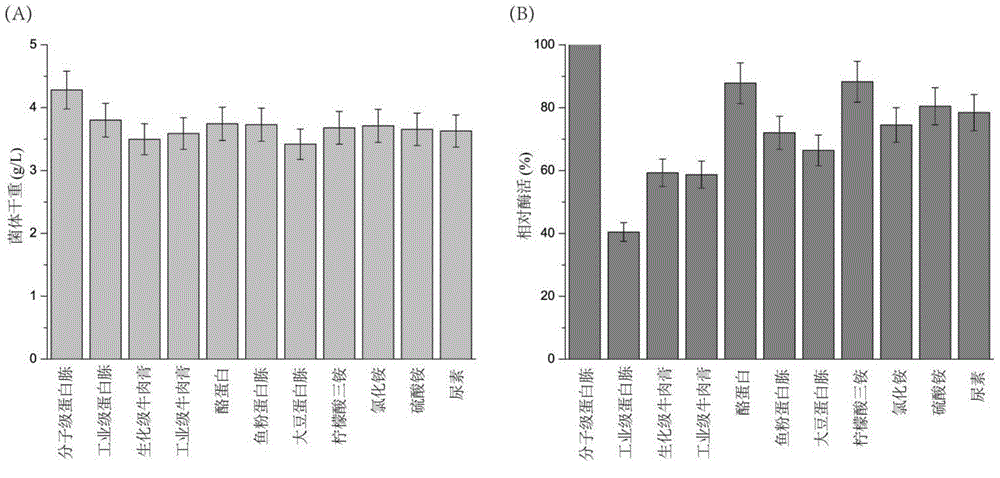
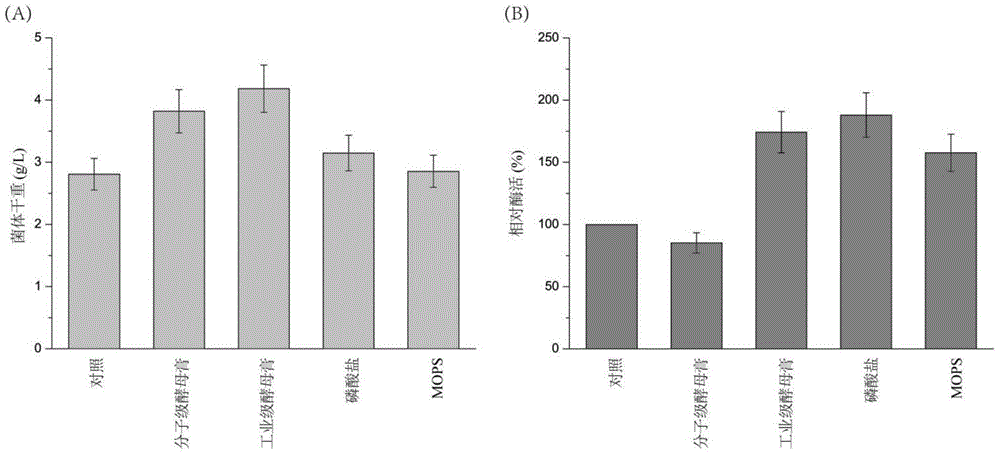
[0026] Embodiment 2 produces SortaseA with different substratum fermentation
[0027]Different carbon sources (dextrin, soluble starch, lactose, galactose, sucrose, fructose, glucose, maltose, glycerin), nitrogen sources (peptone, industrial grade tryptone, industrial grade beef extract, casein, citric acid Triammonium, ammonium chloride, ammonium sulfate, fish powder peptone, beef extract, soybean peptone, urea) replace the corresponding components in the TB medium or add different additives (yeast powder, industrial grade yeast powder, phosphate buffer, MOPS) and metal ions (CaCl 2 、CuSO 4 , FeSO 4 、CoCl 2 , MnSO 4 , LiCl, ZnCl 2 , MgSO 4 , NaCl, NiSO 4 ). In the carbon source and nitrogen source tests, the highest value in the measured enzyme activity is defined as 100%, and the rest of the enzyme activities are relative enzyme activities compared with the highest enzyme activity; The enzyme activity measured during the addition (control) was defined as 100%, and t...
Embodiment 3
[0028] Embodiment 3 Fermentation produces SortaseA with different fermentation conditions
[0029] Fermentation and production of Sortase A with different fermentation temperatures, fermentation times and medium initial pH, Figure 5 , 6 The results showed that when the recombinant Escherichia coli was induced at 16°C, although the growth of the bacteria was slow, the activity of Sortase A was the highest after 4 hours of fermentation; followed by the induction at 20°C for 8 hours, but there was also the problem of slow growth of the bacteria; and then the induction at 30°C 4h, the fermentation temperature is suitable for Escherichia coli growth, and the obtained enzyme activity is also higher ( Figure 5 ). Although the enzyme activity induced by low temperature is the highest, it is not suitable for industrial production due to the defects of slow growth of bacteria and high cost of controlling low temperature. Therefore, the optimum fermentation temperature for enzyme pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com