Tilmicosin premix with fragrance and preparation method of tilmicosin premix
A technology of tilmicosin and premix, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problem of affecting the use of tilmicosin and causing negative psychodynamic effects , Difficulty in taking animals, etc., to achieve the effect of improving drug efficacy, accelerating absorption, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0055] As a preferred embodiment of the present invention, the molar ratio of polyamino acid to cyclodextrin in the polyamino acid-cyclodextrin complex is 1:1-100, more preferably, the molar ratio of polyamino acid to cyclodextrin is 1 : 10-30.
[0056] As a preferred embodiment of the present invention, the polyamino acid is one or more of polyglutamic acid, polyaspartic acid, poly-L-lysine, polylysine, preferably, the The polyamino acid is polyaspartic acid.
[0057] As a preferred embodiment of the present invention, the cyclodextrin is one or more of α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin, preferably, the cyclodextrin is beta-cyclodextrin.
[0058] As a preferred embodiment of the present invention, the binder is starch slurry, polyvinyl alcohol, hydroxypropylmethylcellulose, carboxymethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, etc. One or more mixtures, preferably, the binder is hydroxypropyl methylcellulose.
[0059] As a preferred embod...
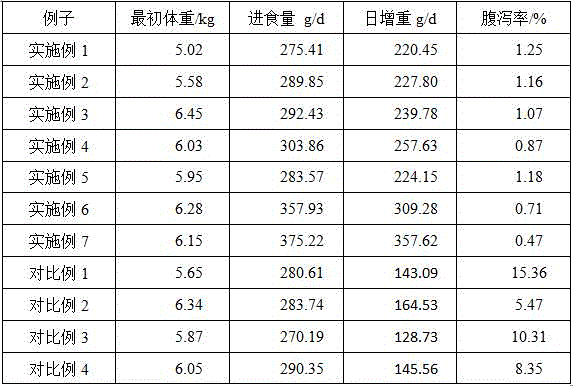
Embodiment 1
[0069] (1) In a 100mL three-necked bottle, add 0.1g of polyaspartic acid, 1-2g of β-cyclodextrin and 50mL of hexamethylphosphoric triamide, stir well at room temperature; After reacting for 15-48 hours, settling and filtering in absolute ethanol to obtain polyaspartic acid-β-cyclodextrin complex;
[0070] (2) 5% by weight of hydroxypropyl methylcellulose, 20% by weight of starch and dextrin mixture, and 15% by weight of polyaspartic acid-β-cyclodextrin complex are added to distilled water, Heat at 50-80°C to dissolve; stir evenly and cool to obtain mixture A;
[0071] (3) Add 20% by weight of tilmicosin to the solution mixture A obtained in step (1), stir for 30-40 minutes, make a soft material, granulate, dry, and sieve.
Embodiment 2
[0073] The preparation method of the polyaspartic acid-β-cyclodextrin complex is the same as in Example 1.
[0074] (1) 5% by weight of hydroxypropyl methylcellulose, 20% by weight of starch and dextrin mixture, and 20% by weight of polyaspartic acid-β-cyclodextrin complex are added to distilled water, Heat at 50-80°C to dissolve; stir evenly and cool to obtain mixture A;
[0075] (2) Add 20% by weight of tilmicosin to the solution mixture A obtained in step (1), stir for 30-40 minutes, make a soft material, granulate, dry, and sieve.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com