Preparation method of Ni0.9Zn0.1O and prepared Ni0.9Zn0.1O and application of Ni0.9Zn0.1O
A hydrothermal reaction, zinc salt technology, applied in electrical components, electrochemical generators, battery electrodes, etc., can solve the problems of not obtaining a single phase structure, high price of cobalt metal, restrictions, etc., and achieve easy industrialization and preparation. Simple method and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
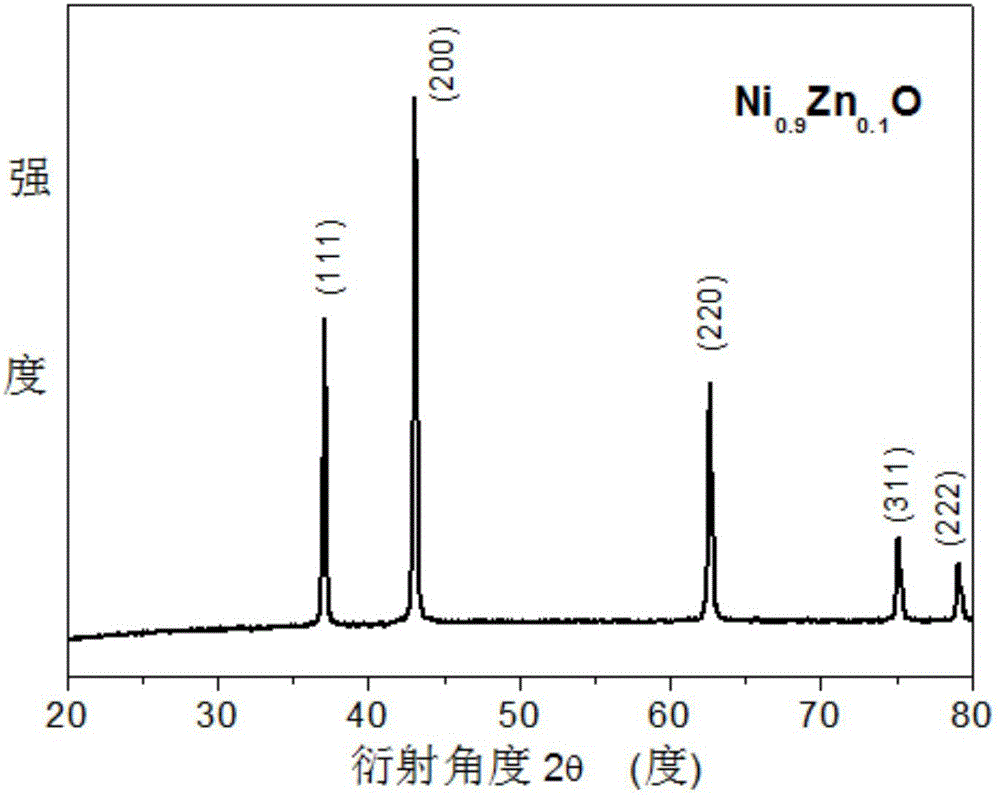
[0032] A kind of nickel-zinc composite oxide Ni of the present invention 0.9 Zn 0.1 The preparation method of O comprises the following steps:
[0033] (1) 11.2g nickel acetate tetrahydrate, 1.1g zinc acetate dihydrate and 0.02g sodium dodecylsulfonate are added to a mixed solvent made of 900ml ethylene glycol and 100ml deionized water, stirred and mixed evenly to obtain Green transparent mixed solution;
[0034] (2) Transfer the mixed solution obtained in step (1) to a 1.5L polytetrafluoroethylene-lined stainless steel reactor, put the reactor into a homogeneous reactor, and perform a hydrothermal reaction at a reaction temperature of 120°C , the reaction time is 10 hours; after the reaction is completed, the obtained product is washed and placed in a vacuum drying oven at 60° C. for 12 hours to obtain a light green organometallic composite precursor;
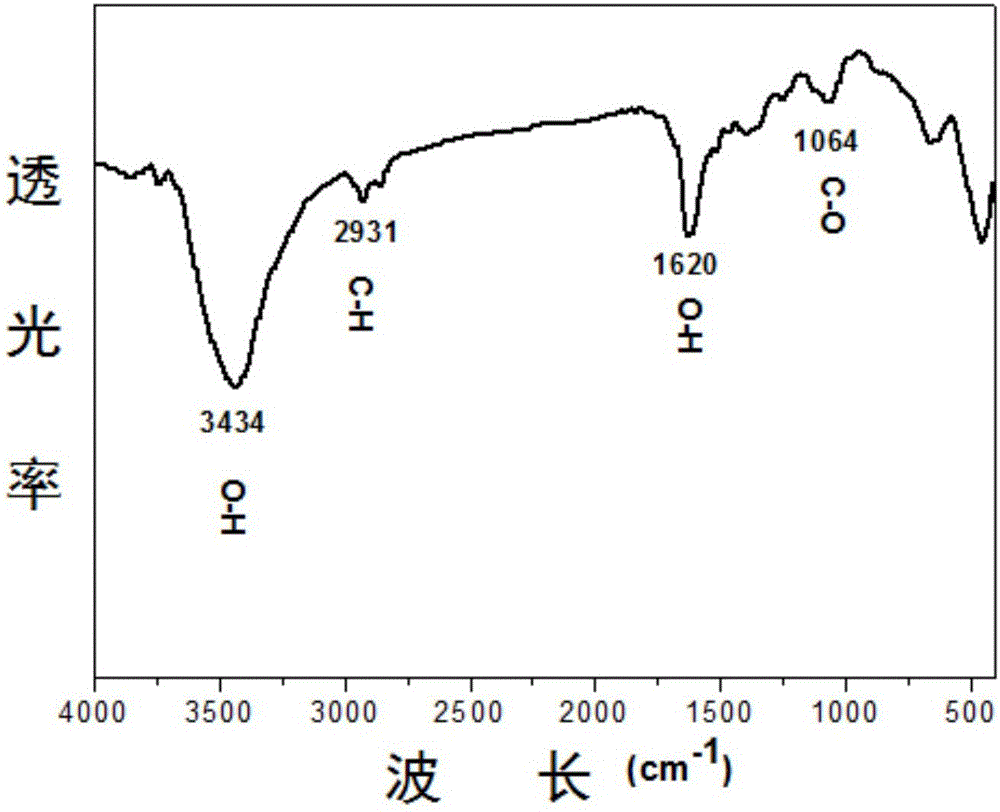
[0035] (3) Put the organometallic composite precursor obtained in step (2) into a crucible and place it in a muffle furna...
Embodiment 2
[0040] A kind of nickel-zinc composite oxide Ni of the present invention 0.9 Zn 0.1 The preparation method of O comprises the following steps:
[0041] (1) Add 11.2g of nickel acetate tetrahydrate, 1.1g of zinc acetate dihydrate and 0.03g of polyvinylpyrrolidone into a mixed solvent made of 900ml of 1,3-propanediol and 100ml of deionized water, stir and mix evenly to obtain a green transparent mixed solution;
[0042] (2) Transfer the mixed solution obtained in step (1) to a 1.5L polytetrafluoroethylene-lined stainless steel reactor, put the reactor into a homogeneous reactor, and perform a hydrothermal reaction at a reaction temperature of 140°C , the reaction time is 10 hours; after the reaction is completed, the obtained product is washed and placed in a vacuum drying oven at 60° C. for 12 hours to obtain a light green organometallic composite precursor;
[0043] (3) Put the organometallic composite precursor obtained in step (2) into a crucible and place it in a muffle ...
Embodiment 3
[0047] A kind of nickel-zinc composite oxide Ni of the present invention 0.9 Zn 0.1 The preparation method of O comprises the following steps:
[0048] (1) 25.28g nickel sulfate heptahydrate, 2.88g zinc sulfate heptahydrate and 0.05g sodium dodecylsulfonate are added to the mixed solvent made of 850ml ethylene glycol and 150ml deionized water, stirred and mixed evenly to obtain Green transparent mixed solution;
[0049] (2) Transfer the mixed solution obtained in step (1) to a 1.5L polytetrafluoroethylene-lined stainless steel reactor, put the reactor into a homogeneous reactor, and perform a hydrothermal reaction at a reaction temperature of 160°C , the reaction time is 10 hours; after the reaction is completed, the obtained product is washed and placed in a vacuum drying oven at 60° C. for 12 hours to obtain a light green organometallic composite precursor;
[0050] (3) Put the organometallic composite precursor obtained in step (2) into a crucible and place it in a muffl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com