Protein medicinal preparation containing beta-glucan as auxiliary material
A pharmaceutical preparation, dextran technology, applied in the biological field, can solve the problems that sucrose is difficult to form a glass state and has no protective effect, and achieve the effects of preventing protein aggregation, enhancing stability, and good water holding capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of soluble β-glucan
[0038] Saccharomyces cerevisiae (Saccharomycescerevisiae, ATCC7752) was used for fermentation and culture to obtain yeast cells.
[0039] Specifically, Saccharomyces cerevisiae is gradually expanded to 1000L-scale fermentation production through shake flask culture. Yeast grows in carbon sources, nitrogen sources, vitamins, inorganic salts and trace elements, and defoamers can also be added to control foam, such as defoamer Antifoam204.
[0040] It is produced in the culture mode of deep aeration combined with fed feeding, and the carbon source glucose or glycerol is selected as the limiting substrate to control fed feeding. During production and fermentation, samples are taken regularly to determine the optical density OD of the fermentation broth 600nm , when OD 600nm After reaching a stable value or a certain value, the fermentation production is terminated. After yeast fermentation and cultivation, use a plate and fra...
Embodiment 2
[0045] Example 2: Liquid and lyophilized pharmaceutical product formulations for parenteral administration according to the invention as follows;
[0046] 1. Preparation of liquid formulations
[0047] Exchange the trastuzumab solution into the corresponding formulation buffer (e.g. 20 mM L-histidine, pH 6.0 or 20 mM L-histidine, 0.02% w / v polysorbate) by ultrafiltration / diafiltration method 20, pH6.0), and concentrated to a certain protein concentration, such as 70mg / ml. It is then diluted to the desired protein concentration with the corresponding formulation buffer. The stabilizer soluble beta-glucan can be added in dissolved form and the surfactant can be added in solution. Finally, the same volume of trastuzumab preparation solution was mixed evenly.
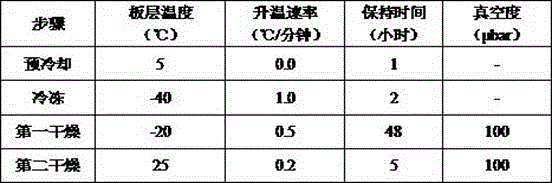
[0048] All formulations were sterilized by sterile filtration through a 0.22 μm filter and dispensed under aseptic conditions into sterile glass vials and sealed with rubber stoppers and aluminum caps. These formulations...
Embodiment 3
[0054] Example 3: Trastuzumab (Trastuzumab) and Pertuzumab (Pertuzumab) Antibody Combination Liquid Formulation
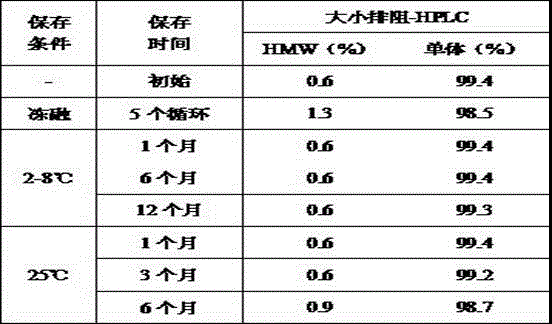
[0055] Formula: 25mg / ml trastuzumab, 25mg / ml pertuzumab, 20mML-histidine, 2% soluble β-glucan, 0.02% Tween20, pH6.5; specific steps are shown in Table 2 ,
[0056] Table 2: Stability Data for Trastuzumab and Pertuzumab Antibody Combination Liquid Formulations
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com