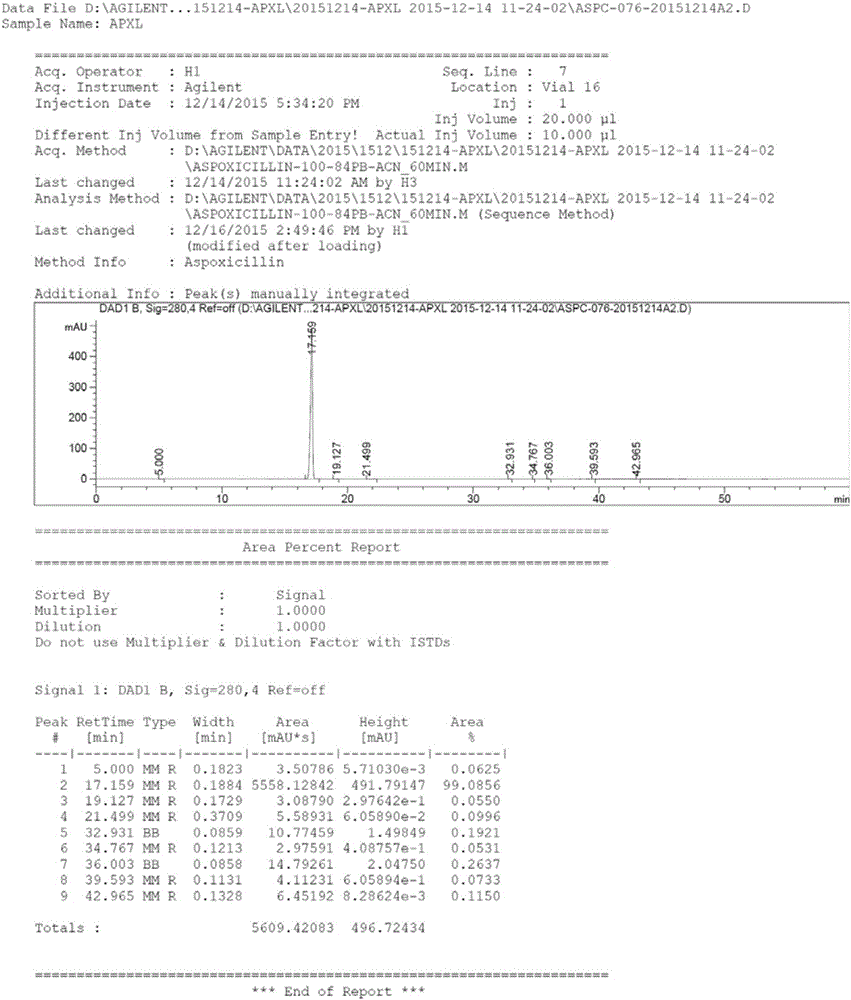
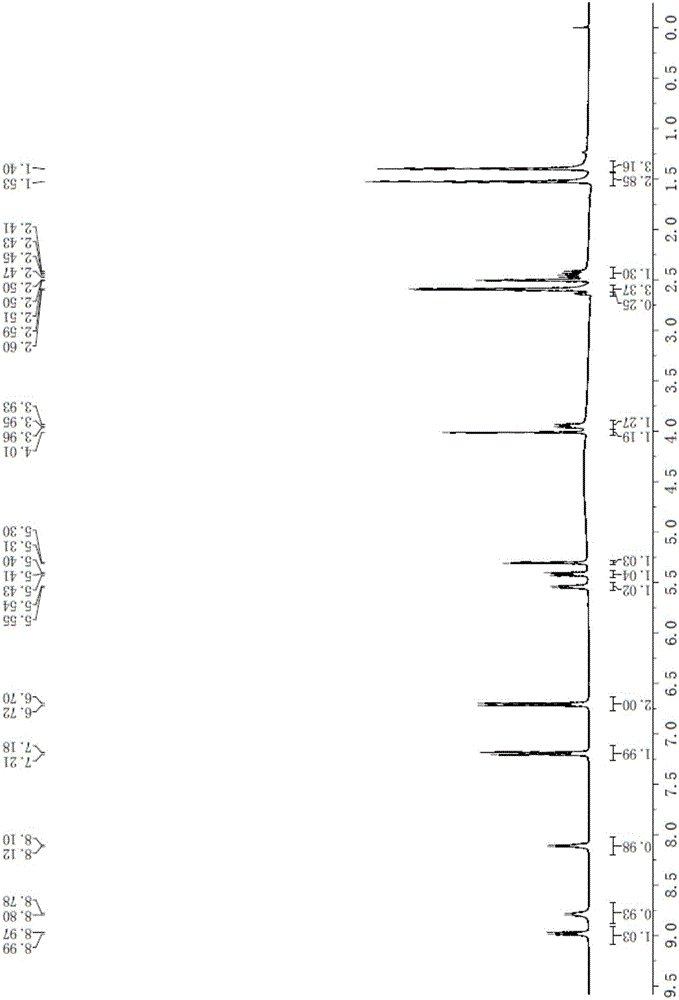
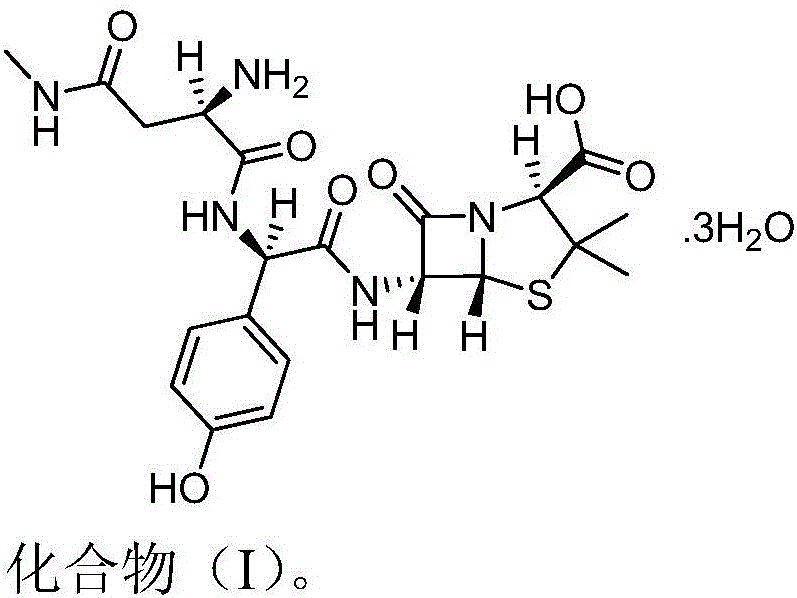
Aspoxicillin synthesis process
A technology of apoxicillin and amoxicillin, which is applied in the field of drug synthesis, can solve the problems of unsuitability for industrial production, poor repeatability, and unstable synthesis process, and achieve the effects of less impurities, convenient post-processing, and shortened reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 20 g of amoxicillin trihydrate, 120 g of anhydrous DCM, 2.25 eq (calculated based on the amount of amoxicillin trihydrate) of TEA, cooled to 0° C., stirred and kept for 1 h to obtain solution A.
[0040] Combine Deng potassium salt (1.5eq of amoxicillin), dichloromethane and catalytic amount of N-methylmorpholine (0.05eq of amoxicillin), stir and cool down to -20°C, and add chlorine dropwise under temperature control -15°C Formic acid ester (1.05eq of Deng potassium salt, after the dropwise addition, react at -15°C for 3 hours to obtain solution B.
[0041]Below -15°C, add solution B dropwise to solution A for 1.5 hours, follow the reaction by HPLC, after the end, remove the dichloromethane solvent by rotary evaporation, add 50ml of acetonitrile, stir to dissolve, add p-toluenesulfonic acid Hydrate (2.2eq), a solid was precipitated, and a crude product of apoxicillin was obtained by filtration. At 0°C, the primary apoxicillin product and the organic solvent isopropanol...
Embodiment 2
[0043] The p-toluenesulfonic acid monohydrate is replaced by citric acid to form a salt, the equivalent weight remains unchanged, the product yield is 85%, and the purity is greater than 99%.
Embodiment 3
[0045] The p-toluenesulfonic acid monohydrate is replaced by salicylic acid to form a salt, the equivalent weight remains unchanged, the product yield is 94%, and the purity is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com