Method for detecting fluorescence or absorbance, method for suppressing background, method for measuring ADP, method for measuring activity of ADP-synthesizing enzyme, and method for measuring activity of glucosyltransferase
An activity measurement and detection method technology, which is applied in the fields of fluorescence or absorbance detection, background suppression, ADP measurement, activity measurement of ADP-generating enzymes, and glycosyltransferase activity measurement, which can solve difficult analysis and detection sensitivity. Low, complicated operation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
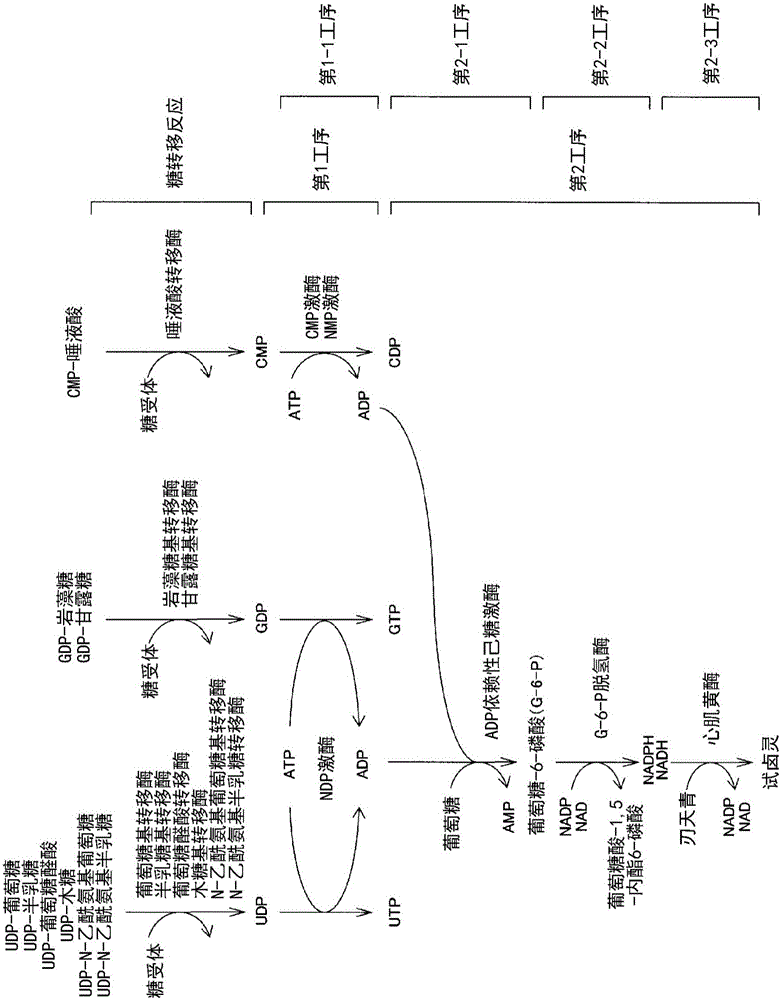
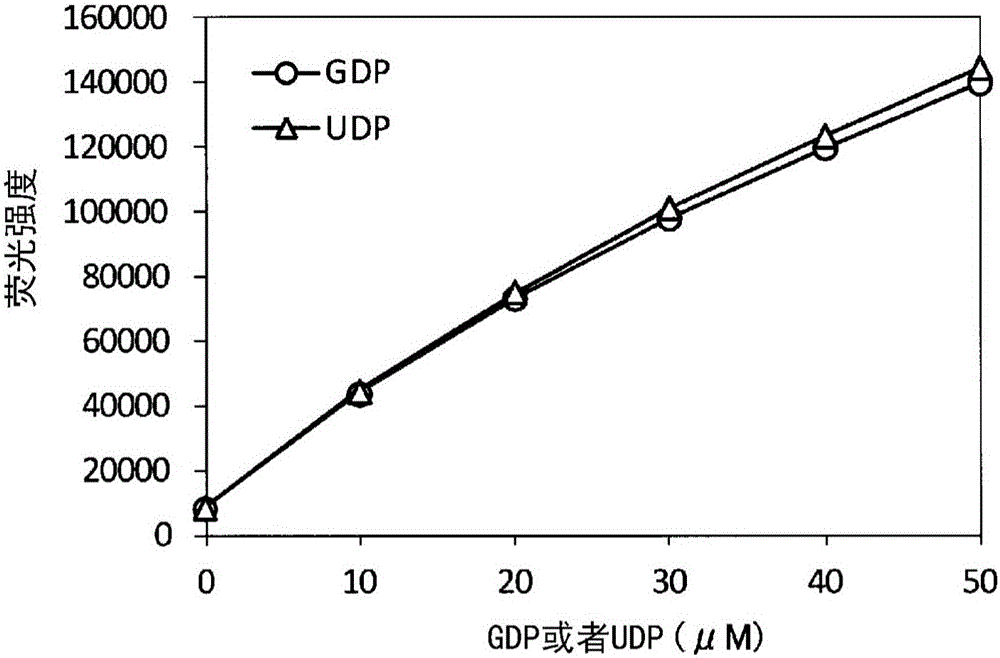
[0296] Use the calibration curve of the GDP of the method of the present invention, UDP
[0297] Calibration curves of GDP and UDP were created using the reactions of the first step and the second step of the present invention.
[0298] As materials, GDP (Wako Pure Chemical Industries, Ltd. catalog number 078-04741), UDP (Wako Pure Chemical Industries, Ltd. catalog number 212-00861), ATP (Wako Pure Chemical Industries, Ltd. catalog number 18-16911), NDP kinase from baker's yeast (Sigma-Aldrich (Sigma-Aldrich) catalog number N0379), glucose (Wako Pure Chemical Industries, Ltd. catalog number 045-31162), ADP-dependent hexokinase (Asahi Kasei Pharmaceutical Co., Ltd. Farm company) catalog number T-93, from thermococcus (Thermococcuslitoralis)), G-6-P dehydrogenase (Oriental yeast industry company (Oriental yeast industry company) catalog number 46857003), diaphorase (especially Nichiko (Unichika Corporation) catalog number B1D111), NADP (Oriental Yeast Industry Corporation (Orie...
Embodiment 2
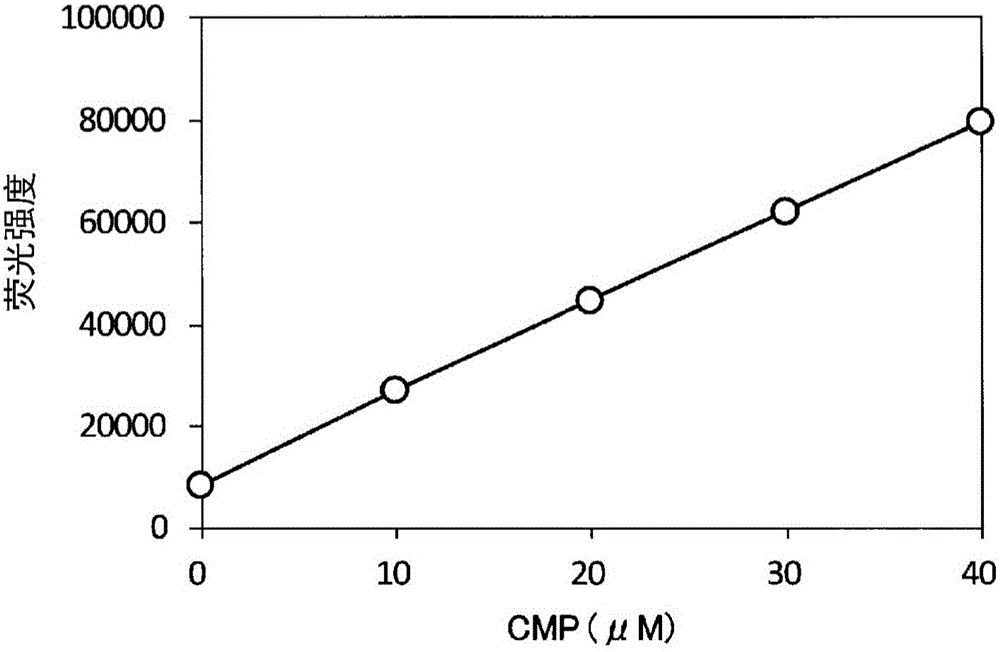
[0309] Calibration curve for CMP using the method of the invention
[0310] A calibration curve of CMP was created using the reactions of the first step and the second step of the present invention.
[0311] As materials, CMP (Wako Pure Chemical Industries, Ltd. catalog number 034-05361), CMP kinase (human CMPK1, Prospec catalog number PKA-002), DTT (Wako Pure Chemical Industries, Ltd. No. 040-29223), N-ethylmaleimide (Wako Pure Chemical Industries, Ltd. Catalog No. 054-02061). Dissolve CMP in buffer F (100 mM Tris-HCl (pH9), 13.5 mM MgCl 2 , 150 mM KCl, 0.1% Triton X-100 (TritonX-100)) to prepare a 0-40 μM solution. Prepare the mixed solution for enzyme coupling according to the following composition.
[0312] Since CMP kinase requires a reducing agent, DTT was used as a reducing agent in the first step, and N-ethylmaleimide was used as an SH reagent for inactivation in the second step.
[0313] Mixture D for the first step
[0314]
[0315] Mixture E for the second s...
Embodiment 3
[0319] Time-dependent changes in fluorochrome
[0320] After performing the reaction in the same manner as in Example 1 using 40 μM of GDP or UDP, the plate was taken out at intervals only during the reaction in the second step for fluorescence measurement. In addition, using 40 μM CMP, the reaction was carried out in the same manner as in Example 2, and only in the reaction in the second step, the plate was taken out at intervals for fluorescence measurement. The results are expressed in Figure 4 . It can be seen from this that, in the case of quantitative GDP or UDP, the reaction of the second step is completed within 25 minutes, and the fluorescence value is kept stably for at least 120 minutes afterwards; in the case of quantitative CDP, the reaction of the second step is completed within 25 minutes. At the end of the minute, there was little change in the fluorescence value until at least 120 minutes after 60 minutes.
[0321] Therefore, in the case of quantitative GD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com