The preparation method of parecoxib
A technology of parecoxib and its compound, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as increased cost, increased reaction steps, and expensive reagents, and achieves the effects of simple operation, mild reaction conditions, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
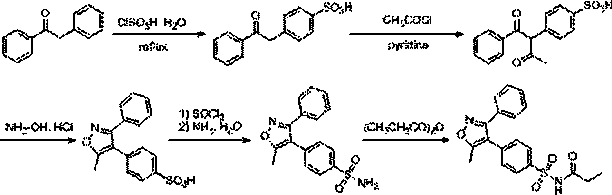
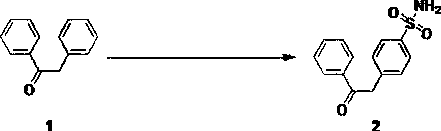
[0027] Example 1 Preparation of 4-(2-oxo-2-phenylethyl)amine benzenesulfonate (compound of formula 2)
[0028] .
[0029] Add 9.8 g of 1,2-benzophenone (compound of formula 1) and 100 mL of dichloromethane into a three-necked round-bottomed flask, stir to dissolve it, then cool down to -10°C, and then slowly add 58.3 g of chlorine under the protection of nitrogen. Sulfonic acid, the temperature is maintained at -5°C~0°C. After the addition, continue to stir at room temperature for 1 hour, then heat and reflux for 5 hours, cool the reaction solution to room temperature, and slowly drop it into ice under stirring. In the water mixture, let stand to separate the layers, collect the organic phase, extract the water phase with dichloromethane (70mL×3), combine the organic phases, dry and concentrate, then add 150 mL of ammonia water, continue stirring for 3 hours, then concentrate to remove water, The solid is washed with 30 mL of isopropanol, then with isopropanol: water: butan...
Embodiment 2
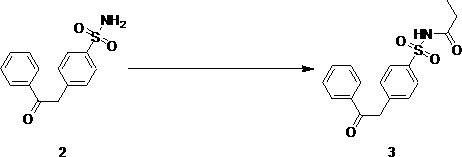
[0031] The preparation of embodiment two [4-(2-oxo-2-phenylethyl) benzenesulfonyl] propanamide (compound of formula 3)
[0032]
[0033] Add 8.25 g of 4-(2-oxo-2-phenylethyl)benzenesulfonic acid amine (compound of formula 2), 33 mL of propionic anhydride, 3.94 g of triethylamine, 4-dimethyl Aminopyridine 0.08 g and tetrahydrofuran 100 mL, stirred and refluxed for 5 hours, after cooling, added 50 mL of saturated aqueous solution of sodium bicarbonate to the mixture, allowed to stand for layers, the organic phase was washed with saturated brine, stood for layers, and collected the organic phase , it is dried and concentrated, and the solid is ethyl acetate: sherwood oil is a 1:3 mixed solvent for stirring and beating for 30 minutes, filters, and the filter cake is washed with ethyl acetate: a 1:3 mixed solvent of sherwood oil, and the dried filter cake obtains White solid 6.95 g, yield 70%.
[0034] 1 H-NMR (400MHz, CD 3 OD-d 4 ): 7.84-7.69(m, 4H), 7.61-7.38(m, 5H), 4.26(...
Embodiment 3
[0035] Example 3 Preparation of [4-(2-oxo-2-phenethyl-1-acetyl)benzenesulfonyl]propanamide (compound of formula 4)
[0036]
[0037] Add 5 g of [4-(2-oxo-2-phenylethyl)benzenesulfonyl]propanamide into 30 mL of pyridine, slowly add 1.4 g of acetyl chloride dropwise under ice-cooling, Continue to stir the reaction, after the TLC method is used to detect that the reaction is complete, add 10 mL of water to the mixed solution, let it stand and filter, the filter cake is successively washed with 30 mL of water, ethyl acetate: petroleum ether as a mixed solvent of 1:6, and dried. 4.2 g of light yellow solid powder was obtained with a yield of 75%.
[0038] 1 H-NMR (400MHz, CD 3 OD-d 4 ): 7.91-7.79(m, 4H), 7.63-7.46(m, 5H), 4.78(s,1H), 2.37(m, 2H), 2.26(s, 3H), 1.14(m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com