Iron series pigment ihleite and preparation method thereof
An iron-based pigment and chemical method technology, applied in chemical instruments and methods, iron sulfate, iron compounds, etc., can solve the problems of poor product quality, high energy consumption, impure color, etc., and achieve less catalyst dosage and particle size. Uniform, bright and pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Jarosite (NaFe 3 (SO 4 ) 2 (OH) 6 ) preparation
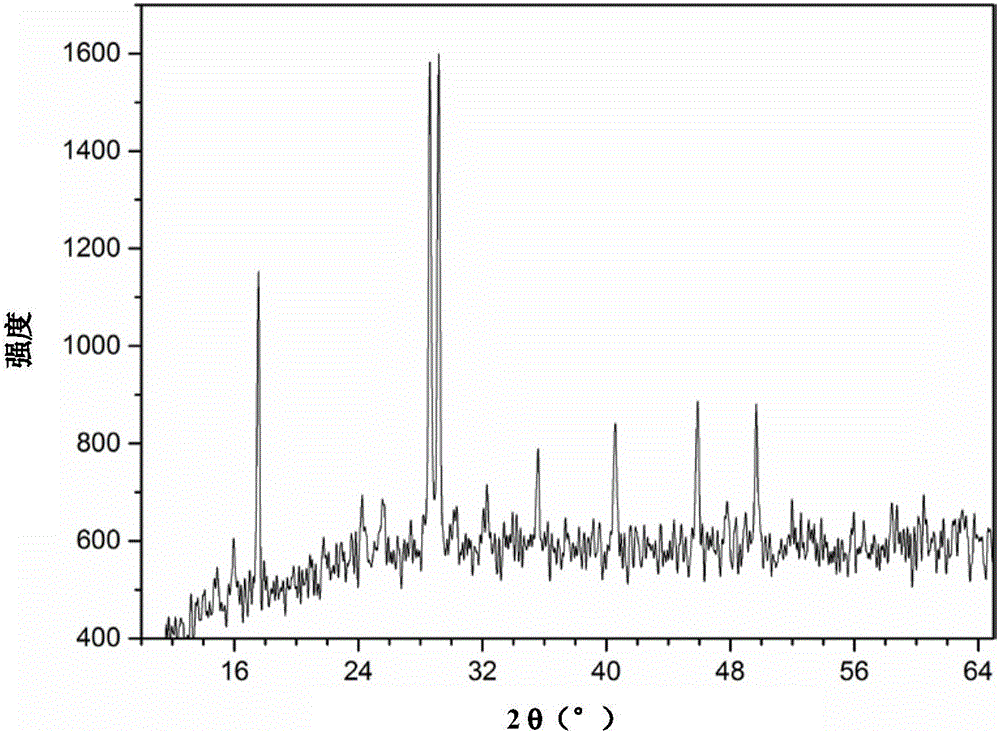
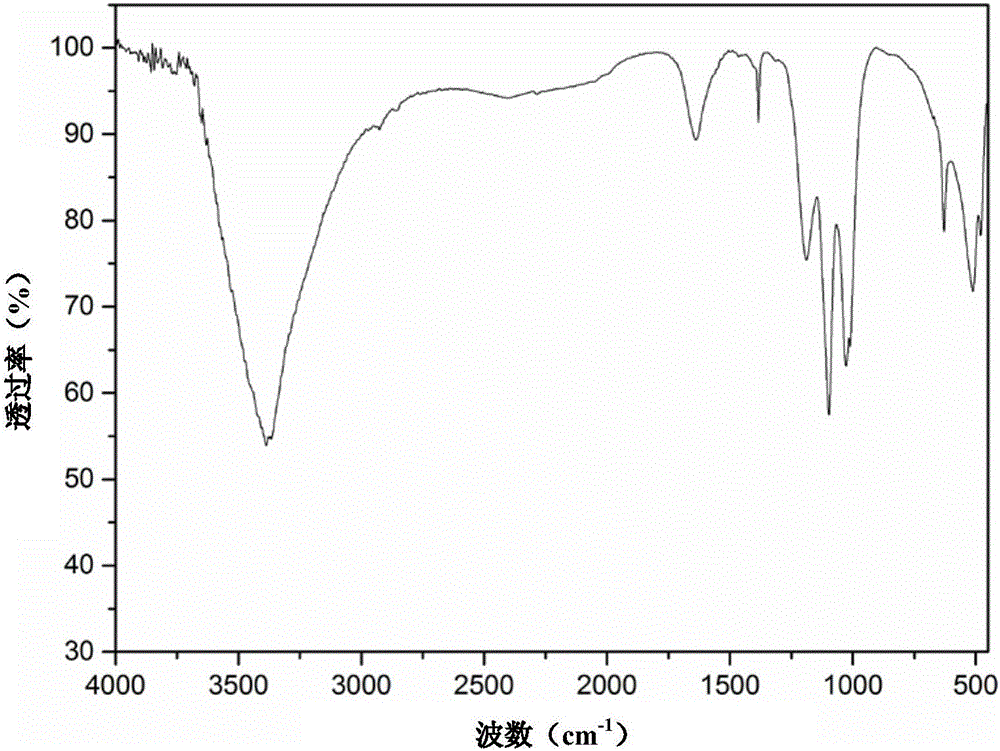
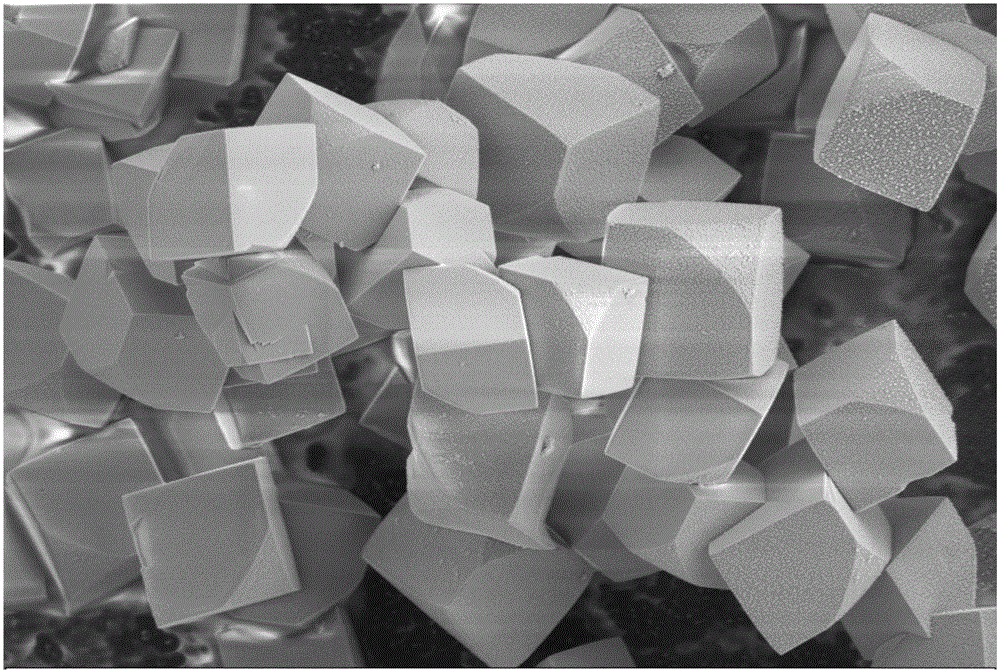
[0034] In the reactor 13.9g FeSO 4 ·7H 2 O and 1.18g of anhydrous sodium sulfate were dissolved in 12.8g of distilled water, and 0.4g of concentrated sulfuric acid was added under stirring. g sodium nitrite to maintain the reaction for 2 hours. After the reaction, the material was naturally cooled and allowed to stand. Then filter, and the jarosite filter cake is washed 3 times with sulfuric acid acidified water with a pH value of 2.0 to remove soluble impurities, then washed 2 times with distilled water, and dried at 95°C to obtain fine particles and uniform size , bright-colored jarosite powder, see XRD pattern figure 1 , FTIR spectrum see figure 2 , SEM image see image 3 .
Embodiment 2
[0035] Embodiment 2: jarosite (KFe 3 (SO 4 ) 2 (OH) 6 )preparation
[0036] In the reactor 27.8g FeSO 4 ·7H 2 O and 4.35g of potassium sulfate were dissolved in 25.6g of distilled water, and 0.75g of concentrated sulfuric acid was added under stirring. Under airtight conditions, air or oxygen was introduced, and the temperature of the reactor was heated to 70°C. Slowly add 0.25g of nitric acid in small amounts for several times. , to maintain the reaction for 2.5 hours. After the reaction, the material was naturally cooled, left to stand, filtered, washed 4 times with acidified water with a pH value of 1.7 to remove soluble impurities, then washed 4 times with distilled water, and dried at 110°C to constant weight. The jarosite powder with fine particles, smooth surface and bright color can be obtained.
Embodiment 3
[0037] Embodiment 3: yellow ammonium jarosite (NH 4 Fe 3 (SO 4 ) 2 (OH) 6 )preparation
[0038] In the reactor 41.7g FeSO 4 ·7H 2 O and 6.6g of ammonium sulfate were dissolved in 32.9g of distilled water, and 1.1g of concentrated sulfuric acid was added under stirring. Under airtight conditions, air or oxygen was introduced, and the temperature of the reactor was heated to 25°C. Slowly added 1.68g of nitric acid , to maintain the reaction for 4 hours. After the reaction, the material was cooled naturally, left to stand, filtered, washed three times with acidified water with a pH value of 1.5 to remove soluble impurities, then washed twice with distilled water, and dried at a drying temperature of 110°C until constant weight. The jarosite powder with fine particles, smooth surface and bright color can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com