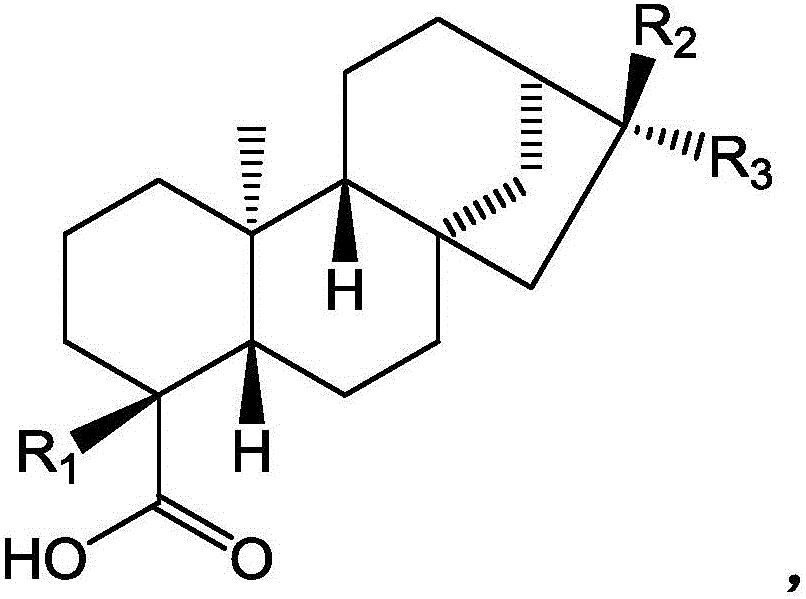
Diterpenoids extracted from herba siegesbeckiae, and preparation method and application thereof
An extraction method and a diterpenoid technology are applied in the field of medicine, and can solve the problems of superinfection of fungi, abuse of antimicrobial drugs, adverse reactions and the like, and achieve the effects of low cost, high production efficiency and simple preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
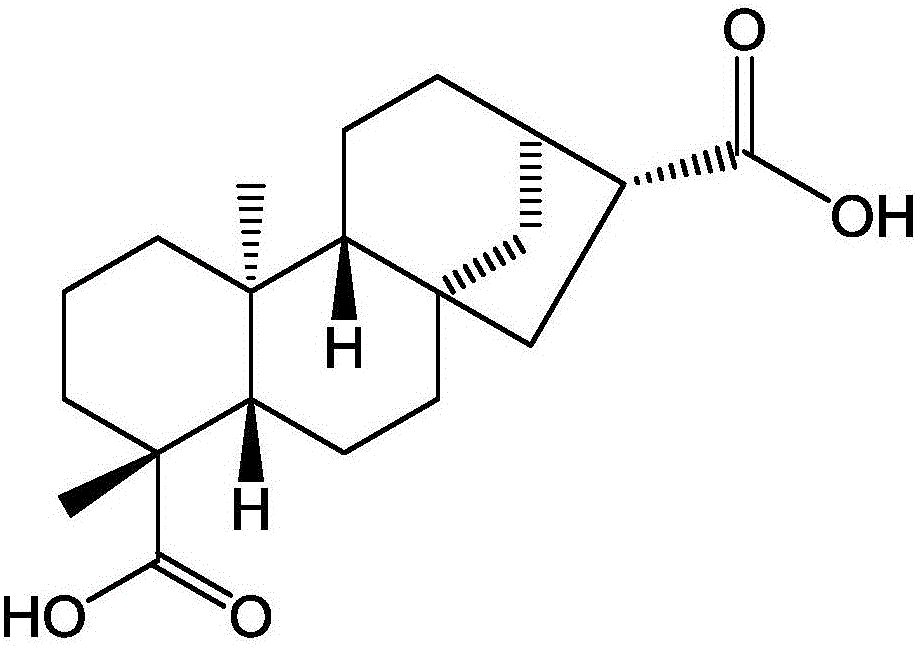
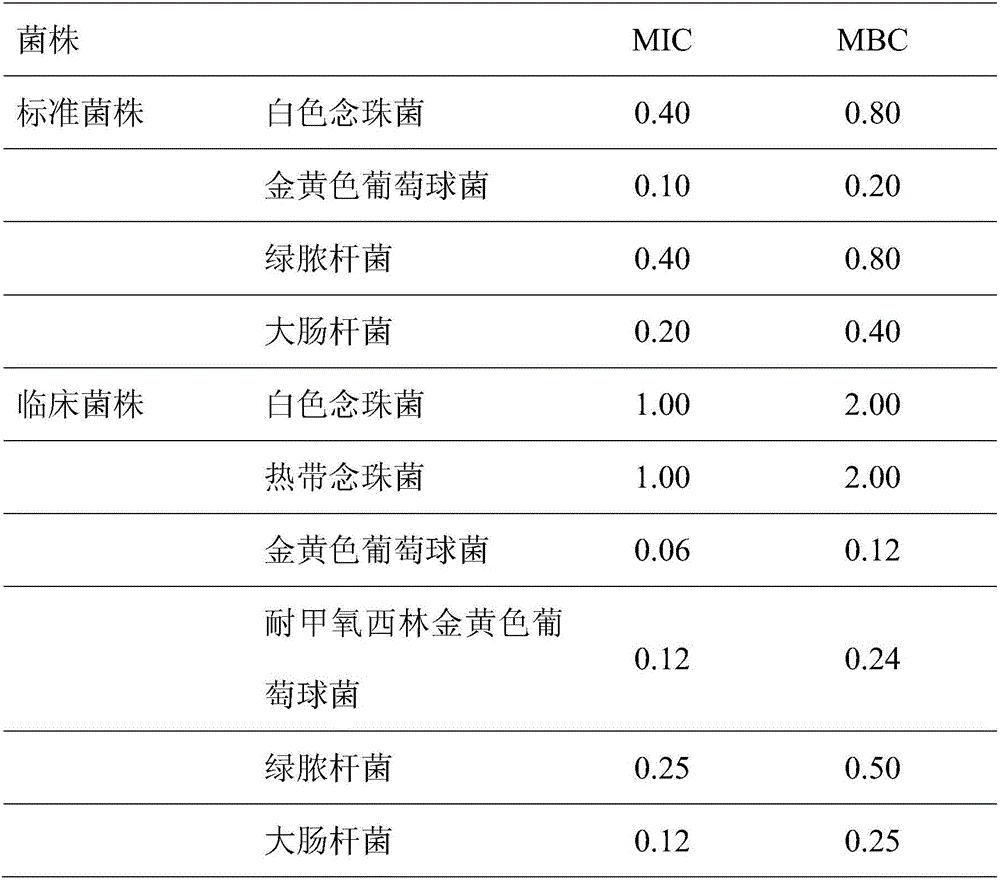
[0030] This example provides the preparation method of the compound ent-16βH-kaurane-17,19-dicarboxylic acid and the measurement method and results of its antibacterial activity.
[0031] Its chemical structural formula is:
[0032]
[0033] Step 1. Extraction: After pulverizing the weed grass, soak it in absolute ethanol, and extract it by ultrasonic wave three times at 25°C for 15, 30 and 45 minutes respectively, filter, collect the filtrate and put it under reduced pressure or normal pressure. Concentrate down to less than 3% to 5% of the volume of the original solution to obtain a concentrated solution;
[0034] Step 2, degreasing: add 0.5 to 1.5 times the volume of water to dissolve the above concentrated solution, extract with an equal volume of n-hexane or cyclohexane or petroleum ether, discard the extract, and collect the remaining water;
[0035] Step 3, separation: Concentrate the remaining aqueous solution obtained in the previous step to its volume of 1% to 3%...
Embodiment 2
[0044] This example provides the preparation method of the compound ent-16α, 17-dihydroxykaurene-19-carboxylic acid and the measurement method and results of its antibacterial activity.
[0045] Its chemical structure is:
[0046]
[0047] Preparation:
[0048] Step 1. Extraction: After pulverizing the weed grass, heat and reflux at 45-60°C for 2 hours with an ethanol aqueous solution with a concentration of 85-95% by volume, extract 3 times, filter, and collect the filtrate under reduced pressure or normal pressure Concentrate down to less than 3% to 5% of the volume of the original solution to obtain a concentrated solution;
[0049] Step 2, degreasing: add 0.5 to 1.5 times the volume of water to dissolve the above concentrated solution, extract with an equal volume of n-hexane or cyclohexane or petroleum ether, discard the extract, and collect the remaining water;
[0050] Step 3, separation: Concentrate the remaining aqueous solution obtained in the previous step to i...
Embodiment 3
[0059] This example provides the preparation method of the compound ent-16α, 17, 18-trihydroxykaurene-19-carboxylic acid and the measurement method and results of its antibacterial activity.
[0060] Its chemical structure is:
[0061]
[0062] Preparation:
[0063] Step 1. Extraction: After pulverizing the weed grass, heat and reflux with anhydrous methanol solution at 45-60°C for 2 hours, extract 3 times, filter, collect the filtrate and concentrate it to less than the volume of the original solution under reduced pressure or normal pressure 3% to 5%, to obtain a concentrated solution;
[0064] Step 2, degreasing: add 0.5 to 1.5 times the volume of water to dissolve the above concentrated solution, extract with an equal volume of n-hexane or cyclohexane or petroleum ether, discard the extract, and collect the remaining water;
[0065] Step 3, separation: Concentrate the remaining aqueous solution obtained in the previous step to its volume of 1% to 3%, and then carry ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com