Photocatalyst for preparation of hydrogen and corresponding aldehyde and ketone by means of alcohol decomposition
A photocatalyst and alcohol decomposition technology, which is applied in the direction of physical/chemical process catalyst, dehydrogenation preparation, carbon-based compound preparation, etc., can solve the problems of low reaction selectivity, poor product selectivity, over-oxidation, etc., and achieve high purity and easy Recycling, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
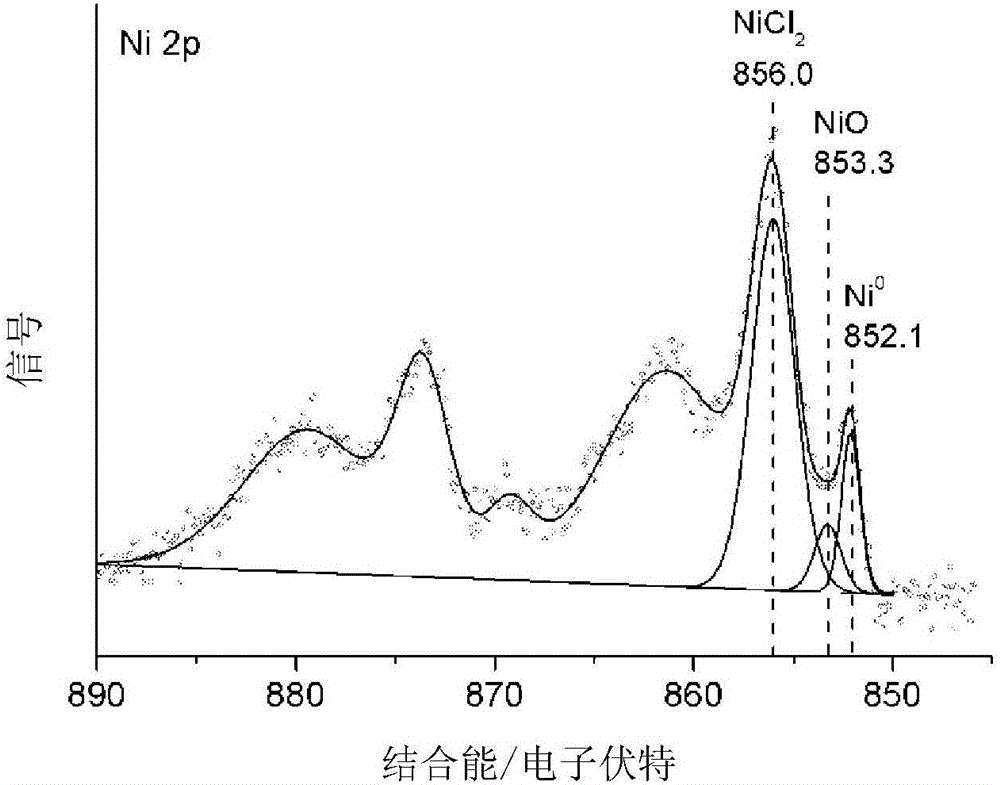
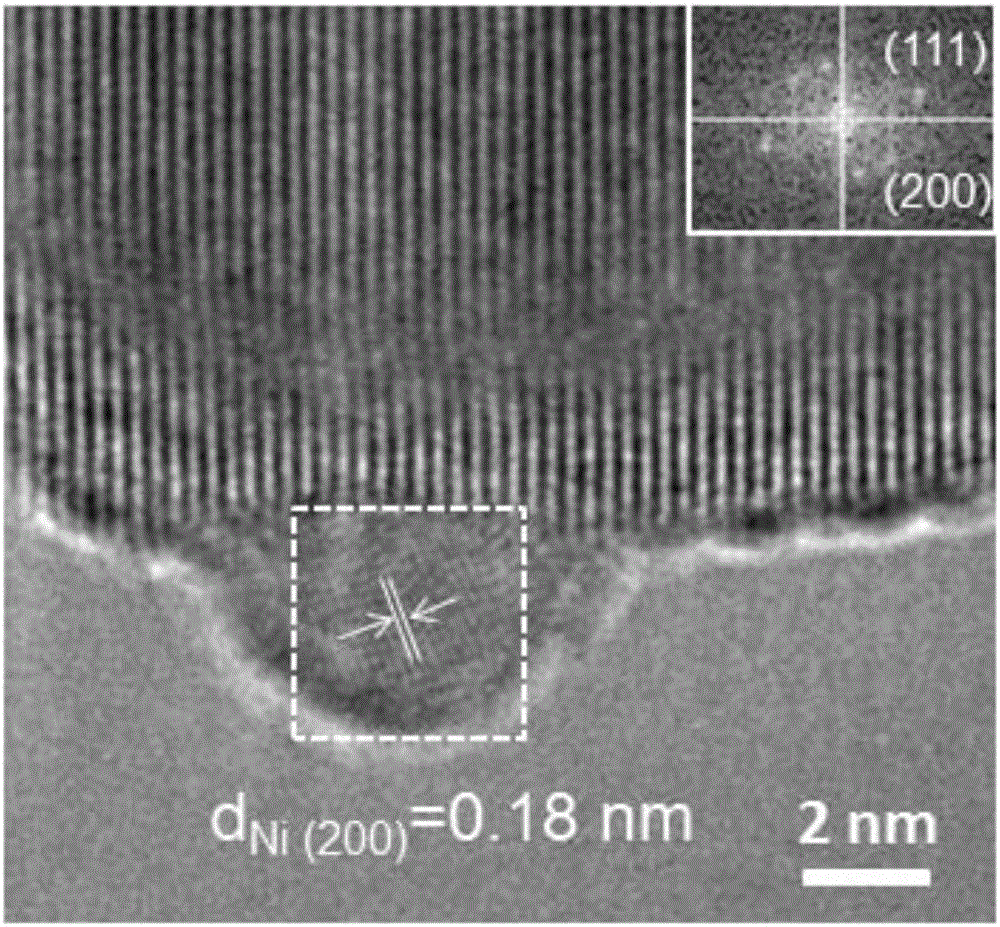
Image
Examples
Embodiment 1
[0023] 1) 7 mg of cadmium sulfide is mixed with 5 mg of nickel chloride hexahydrate in methanol;
[0024] 2) Deoxygenate the system, and then irradiate with visible light (wavelength 420-800 nanometers) for 1 hour in an argon atmosphere under stirring to obtain a catalyst;
[0025] 3) Remove the supernatant, wash the catalyst with methanol, and dry it in vacuum at room temperature;
[0026] 4) Add 5 ml of methanol as a raw material, illuminate with a 300-watt xenon lamp for 24 hours, and generate 27 ml of hydrogen gas.
[0027] CH 3 OH=HCHO+H 2
[0028] The change of hydrogen production volume with the light time is as follows: image 3 As shown, it can be seen that the amount of hydrogen gas produced increases with the increase of illumination time. The amount of formaldehyde in the solution after the reaction was analyzed by gas chromatography, and the molar ratio of the amount of hydrogen obtained to the amount of formaldehyde was 1.0, indicating that the reaction was ...
Embodiment 2
[0030] Press the condition of embodiment 1, change raw material into 5 milliliters of ethanol, the amount of catalyst is the same as embodiment 1, illuminate 24 hours, produce 38 milliliters of hydrogen, the mol ratio of the amount of hydrogen obtained and the amount of acetaldehyde is 1.0.
Embodiment 3
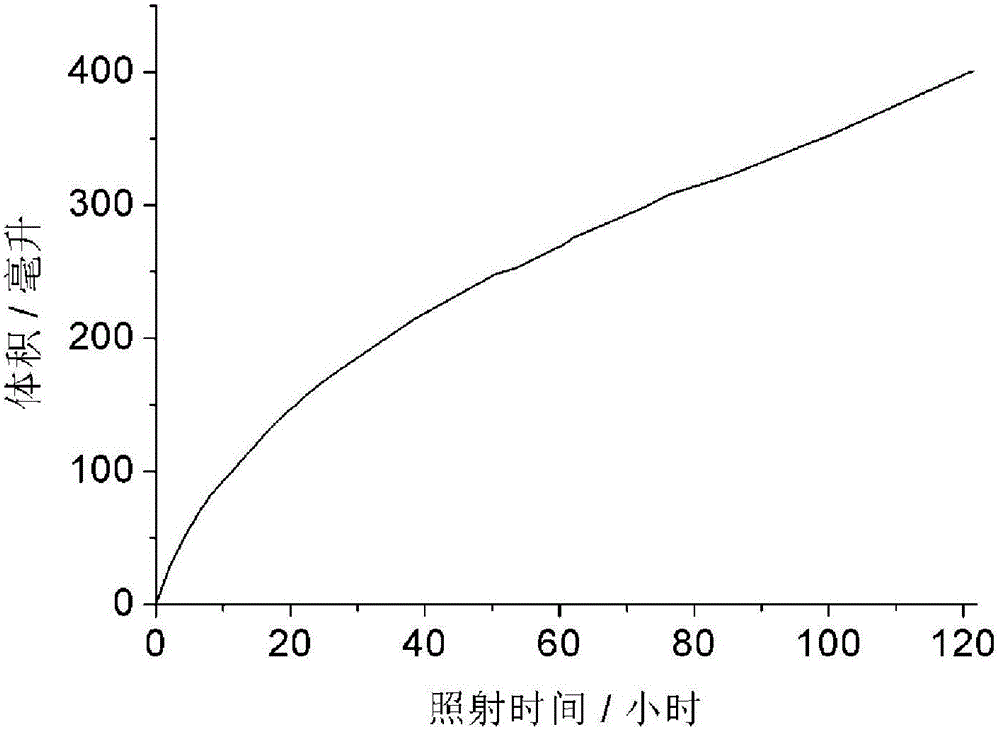
[0032] Press the condition of embodiment 1, change raw material into 5 milliliters of isopropanols, the amount of catalyst is the same as embodiment 1, light 24 hours, produce 165 milliliters of hydrogen, the mol ratio of the amount of hydrogen obtained and the amount of acetone is 1.0. Figure 4 It is the relationship diagram of the hydrogen production volume changing with the illumination time during long-term continuous irradiation in this embodiment. It can be seen from the figure that the photocatalyst can continuously produce hydrogen within 121 hours, indicating that the catalyst has good stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com