Preparation method of sustained-release microparticles, prepared sustained-release microparticles and applications thereof
A technology for slow-release microparticles and microparticles, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., and can solve problems such as denaturation, aggregation and precipitation of active substances, and unstable release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
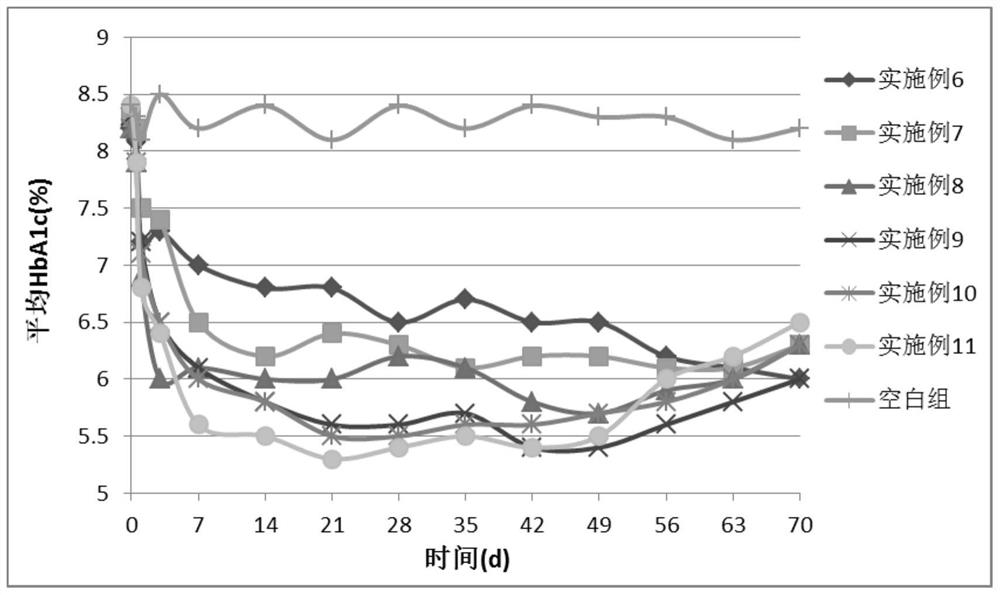
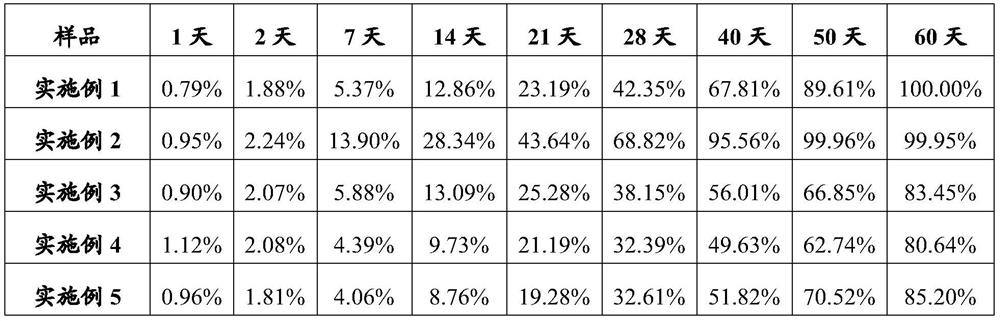
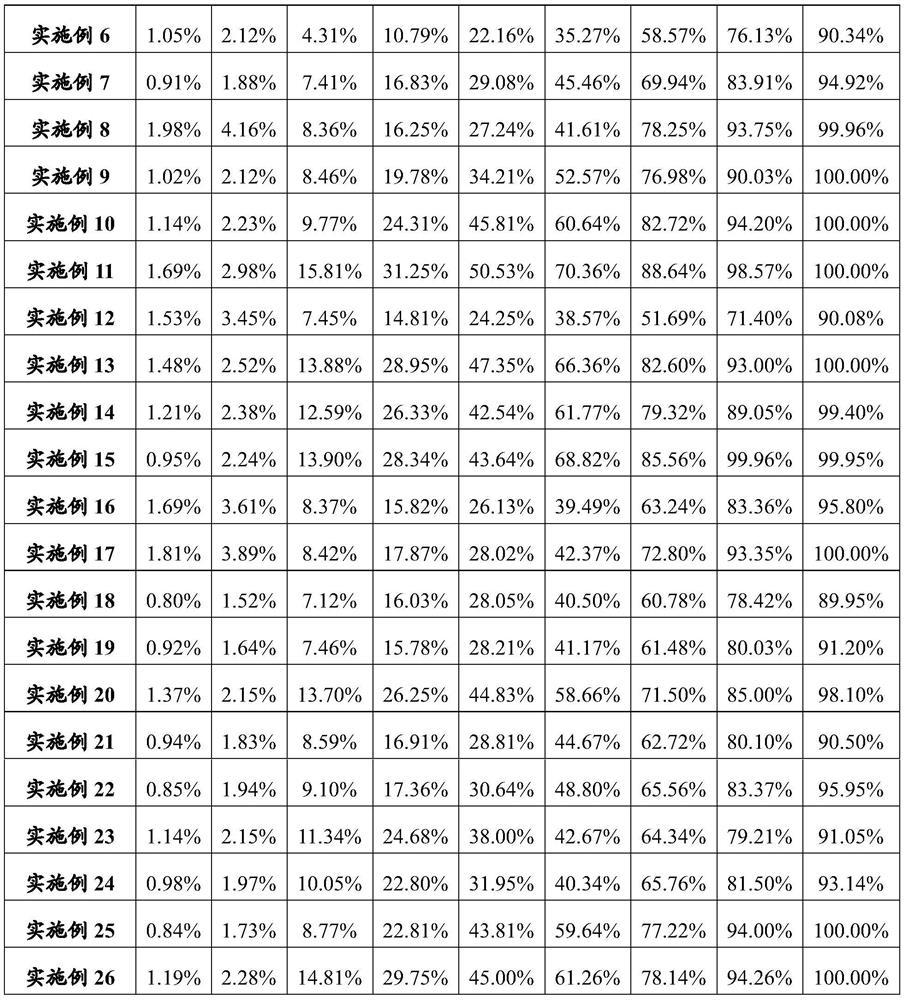
Examples
Embodiment 1
[0104] Example 1 Preparation of albiglutide / PLGA microparticles
[0105] (1) Preparation of solid dispersion
[0106] Dissolve 0.90g PLGA (molecular weight 25kDa, monomer ratio 65 / 35, carboxyl-terminated) in about 6.00mL glacial acetic acid, then add 0.10g albiglutide acetate, dissolve under vortex, and then slowly pour into a stirring In water ether (6°C), a white precipitate was produced, collected the white precipitate and extracted about 5 times with anhydrous ether, dried in a vacuum oven for 24 hours (10°C) after collecting the precipitate, to obtain a solid dispersion.
[0107] (II) Preparation of microparticles
[0108] The solid dispersion obtained in step I was uniformly dispersed in about 6.00 g of dichloromethane to obtain an internal oil phase, and then the internal oil phase was injected into 230 mL of 0.05% (w / w) lecithin / peanut oil that had been pre-warmed to about 4°C solution, and use a high-speed homogenizer to prepare S / O / O emulsion (rotor speed about 3...
Embodiment 2
[0109] Example 2 Preparation of dulaglutide / PLGA microparticles
[0110](1) Preparation of solid dispersion
[0111] Dissolve 0.95g PLGA (molecular weight 30kDa, monomer ratio 50 / 50, carboxyl-terminated) in about 7.92mL acetonitrile, then add 0.05g dulaglutide acetate, dissolve under vortex, and then slowly pour into the cyclohexane under stirring In alkanes (6°C), a white precipitate was produced, which was collected and extracted about 5 times with cyclohexane. After the collected precipitate was dried in a vacuum oven for 24 hours (10°C), a solid dispersion was obtained.
[0112] (II) Preparation of microparticles
[0113] The solid dispersion obtained in step I was uniformly dispersed in about 7.92 g of chloroform to obtain an internal oil phase, and then the internal oil phase was injected into 420 mL of a 0.1% (w / w) lecithin / liquid paraffin solution that had been pre-warmed to about 5°C , and use a high-speed homogenizer to prepare S / O / O emulsion (rotor speed about 3...
Embodiment 3
[0114] Example 3 Preparation of follicle stimulating hormone / PLA microparticles
[0115] (1) Preparation of solid dispersion
[0116] Dissolve 0.97g PLA (molecular weight 20kDa, terminal ester group) in about 5.39mL dimethyl sulfoxide, then add 0.03g follicle-stimulating hormone acetate, 0.05g xylitol and 0.03g zinc chloride, dissolve under vortex, Then it is slowly injected into n-hexane (8°C) under stirring to produce a white precipitate, which is collected and extracted about 5 times with n-hexane, and dried in a vacuum oven for 24 hours (10°C) after the precipitate is collected to obtain Solid dispersion.
[0117] (II) Preparation of microparticles
[0118] The solid dispersion obtained in step I was uniformly dispersed in a mixed solution of about 5.39 g of dichloromethane and chloroform to obtain an internal oil phase, and then the internal oil phase was injected into 410 mL of 0.25% (w / w ) lecithin / soybean oil solution, and emulsified using a turbine homomixer to p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com