Fluorescent aliphatic polyamide imide as well as preparation method and application thereof
A polyamide-imide and aliphatic technology, which is applied in the field of fluorescent aliphatic polyamide-imide and its preparation, can solve the problems of no report on fluorescent aliphatic polyamide-imide, difficult processing and molding of aromatic PAI, etc. , to achieve the effects of wide application range, short reaction time and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Synthesis of Intermediate Thiolactone-Maleimide Monomer (Ⅱ)
[0057]
[0058] (1) Synthesis of 2-bromo-2-methyl-nitrogen-(2-oxotetrahydrothiophen-3-yl)propionamide (c)
[0059]
[0060] Homocysteine thiolactone (7.10g, 46.3mmol) and triethylamine (11.2g, 110.9mmol) were dissolved in 150mL of chloroform, placed in an ice bath, and argon flowed. 2-Bromoisobutyryl bromide (12.65 g, 55.5 mmol) was slowly dropped into the reaction solution, and stirred overnight at room temperature. After the reaction, the reaction solution was diluted with dichloromethane (150 mL), filtered and washed with water (60 mL*3). Combined organic phases, anhydrous MgSO 4 After drying and filtering, a light yellow oily liquid was obtained. After column chromatography (ethyl acetate / n-hexane=1:2), white crystals were obtained. Yield: 49.7%.
[0061] The structural confirmation data is as follows: 1 HNMR (300MHz, CDCl 3 , ppm) δ7.04(s, 1H), 4.45(dt, J=13.0Hz, J=6.5Hz, 1H), 3....
Embodiment 2
[0074] Example 2 Synthesis of Fluorescent Aliphatic Polyamide-imide PAI1
[0075]
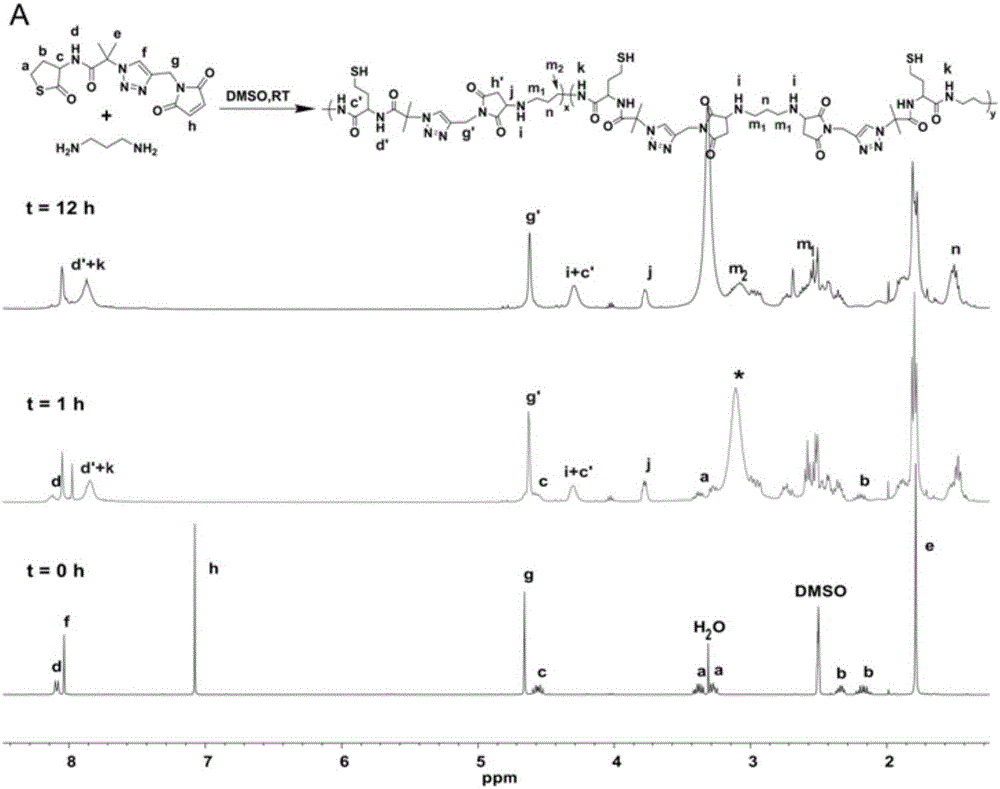
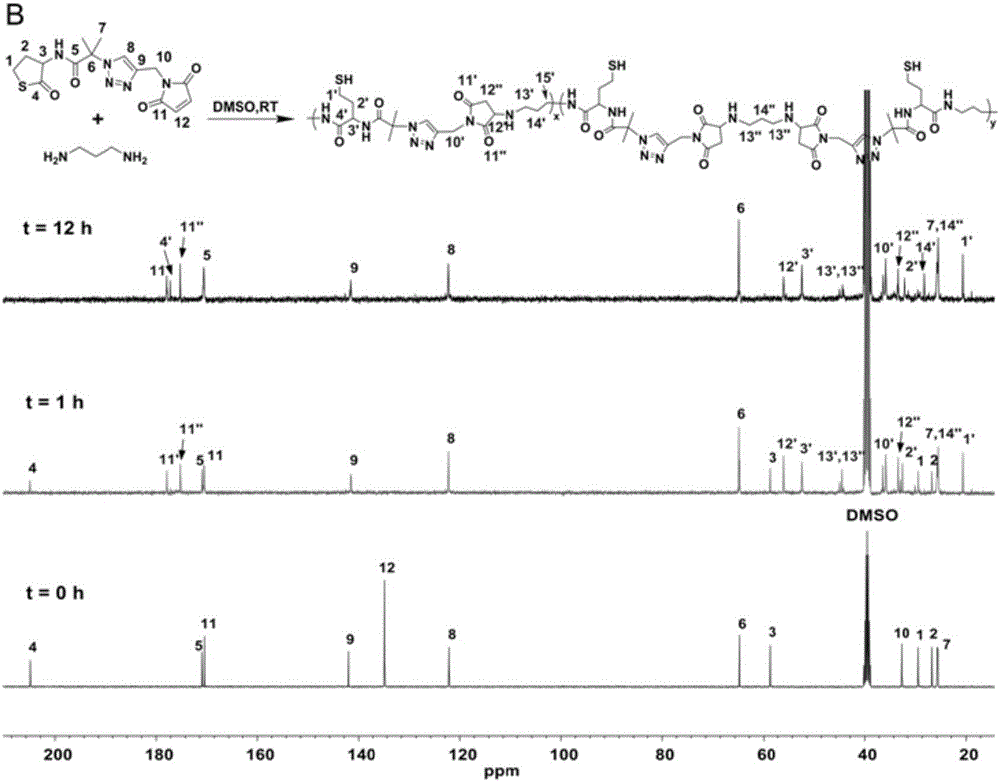
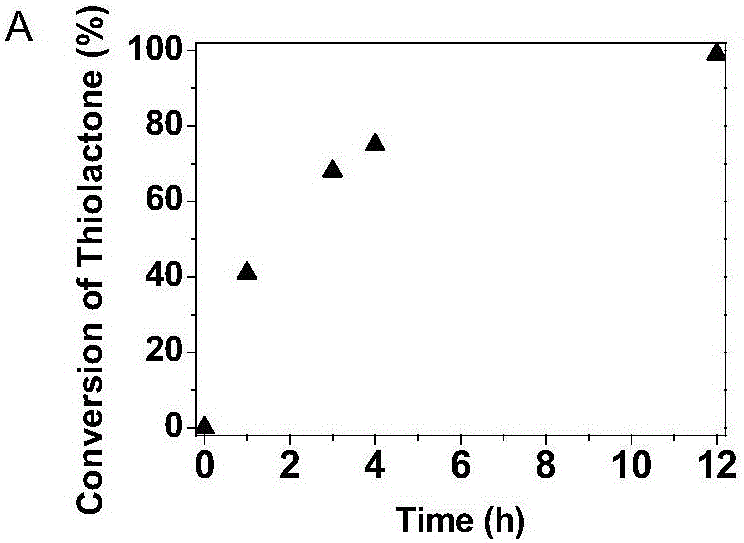
[0076] Polycondensation reaction: thiolactone-maleimide monomer (36.3mg, 0.1mmol) was dissolved in 1mL DMSO, argon flowed, then 1,3-propylenediamine (7.4mg, 0.1mmol) was added, stirred at room temperature , dynamic NMR tracking. After the reaction, the reaction solution was precipitated in acetone and dried in vacuum for 3 hours.
[0077] The equivalent reaction of thiolactone-maleimide and 1,3-propanediamine does not need to add a base as a catalyst. 1,3-Propanediamine will undergo ring-opening reaction with thiolactone and Michael addition reaction with maleimide at the same time. We followed the reaction with NMR, and the experimental results are shown in Figure 1(a) and Figure 1(b).
[0078] By Fig. 1 (a) and Fig. 1 (b) as can be seen, the double bond proton signal peak (δ=7.08ppm, h) of maleimide and the methine proton signal peak of homocysteine thiolactone (δ=7.08ppm, h) gradu...
Embodiment 3
[0081] Example 3 Synthesis of Fluorescent Aliphatic Polyamide-imide PAI2
[0082]
[0083] Polycondensation reaction: thiolactone-maleimide monomer (36.3mg, 0.1mmol) was dissolved in 1mL DMSO, and argon was added, then 2,2-dimethyl-1,3-propylenediamine (0.1 mmol), stirring at room temperature, and dynamic NMR tracking. After the reaction, the reaction solution was precipitated in acetone and dried in vacuum for 3 hours.
[0084] Structure confirmation data such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com