Rare earth luminescence catalyst, and preparation method and applications thereof
A rare earth luminescence and catalyst technology, which is applied in catalytic reactions, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of complex preparation and unsatisfactory catalytic effects, and achieve simple synthesis methods, low production costs, and good performance. The effect of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
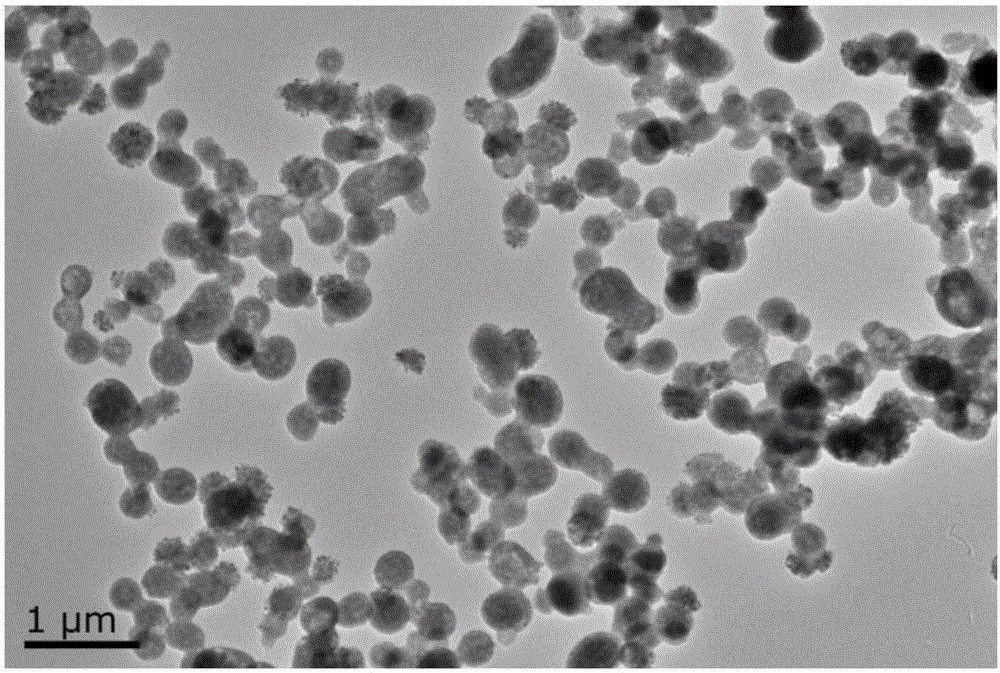
[0028] The preparation method of the rare earth luminescent catalyst Tb-Cu-PA nanoparticles of the present invention specifically comprises the following steps:
[0029] Step 1, to 5mL N,N-dimethylformamide, add 2mL of Tb(NO 3 ) 3 aqueous solution and 2 mL of 20 mM Cu(NO 3 ) 2 Aqueous solution, mixed uniformly to obtain mixed solution A; then, under constant stirring, 2 mL of N,N-dimethylformamide solution dissolved with isophthalic acid was added to mixed solution A to obtain mixed solution B; dissolved with isophthalic acid The N, N-dimethylformamide solution of phthalic acid has a concentration of isophthalic acid of 40 mM;
[0030] Step 2, the mixed solution B of step 1 was stirred and reacted at room temperature for 20 minutes, transferred to a polytetrafluoroethylene-lined reactor, reacted at 160°C for 3 hours, cooled naturally to room temperature, and centrifuged to collect the resulting light green precipitate; The precipitate was washed twice with absolute ethanol...
Embodiment 2
[0035] The application of the rare earth luminescent catalyst Tb-Cu-PA nanoparticles prepared in Example 1 in the reaction of hydrogen peroxide and ascorbic acid:
[0036] Add 10 μL of HO to 960 μL of HAc-NaAc buffer (10 mM, pH 5.05) 2 o 2 Solution (200mM) and 10μL ascorbic acid (200mM), mix well, then add 20μL Tb-Cu-PA suspension (35mg / mL), react at room temperature for 20min, and then measure their fluorescence intensity under 310nm excitation.
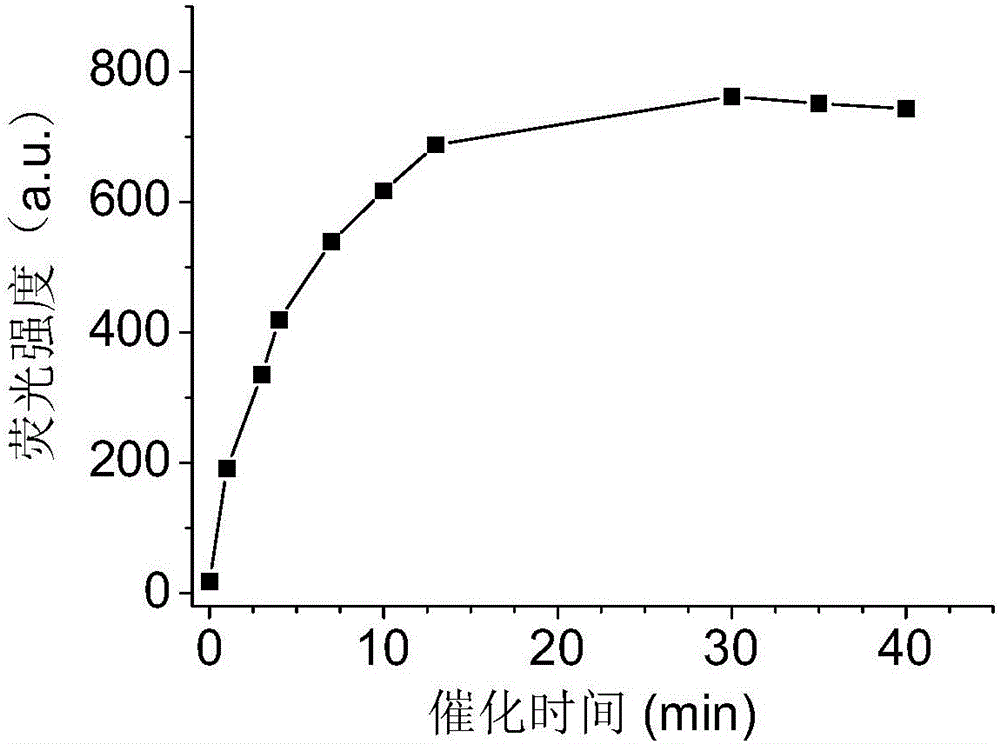
[0037] figure 2 The luminescence intensity figure of the rare earth luminescent catalyst Tb-Cu-PA that is prepared in embodiment 1 in the process of catalyzing hydrogen peroxide and ascorbic acid reaction; In the presence of the catalyst Tb-Cu-PA, fluorescence appeared in the solution within a few minutes, and the fluorescence intensity gradually became stronger as the reaction progressed until it stopped changing, indicating that the reaction was complete.
Embodiment 3
[0039] The application of the rare earth luminescence catalyst Tb-Cu-PA nanoparticle that embodiment 1 makes aspect the hydrogen peroxide concentration in the measurement solution:
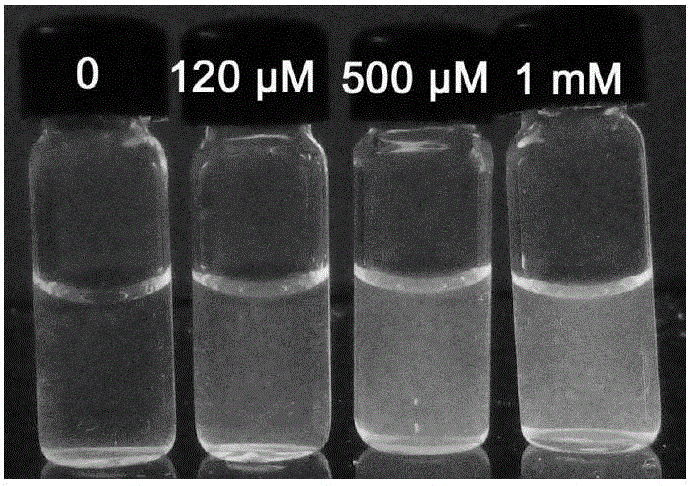
[0040] Add 20 μL of Tb-Cu-PA suspension (35 mg / mL) and 10 μL of ascorbic acid (200 mM) to 960 μL of HAc-NaAc buffer (10 mM, pH 5.05), mix well, and then add different volumes of H 2 o 2 solution (5mM) and ultrapure water to the above mixture, making H 2 o 2 The final concentrations were 0, 1, 100, 240, 360 and 500 μM, respectively. After these mixed solutions were reacted at room temperature for 20 minutes, their fluorescence spectra were measured under 310nm excitation to obtain the working curve of fluorescence intensity and hydrogen peroxide concentration, and the fluorescence color maps containing hydrogen peroxide solutions with different concentrations were taken under a 302nm ultraviolet lamp.
[0041] Add 20 μL of Tb-Cu-PA suspension (35 mg / mL) and 10 μL of ascorbic acid (200 mM) to 960...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com