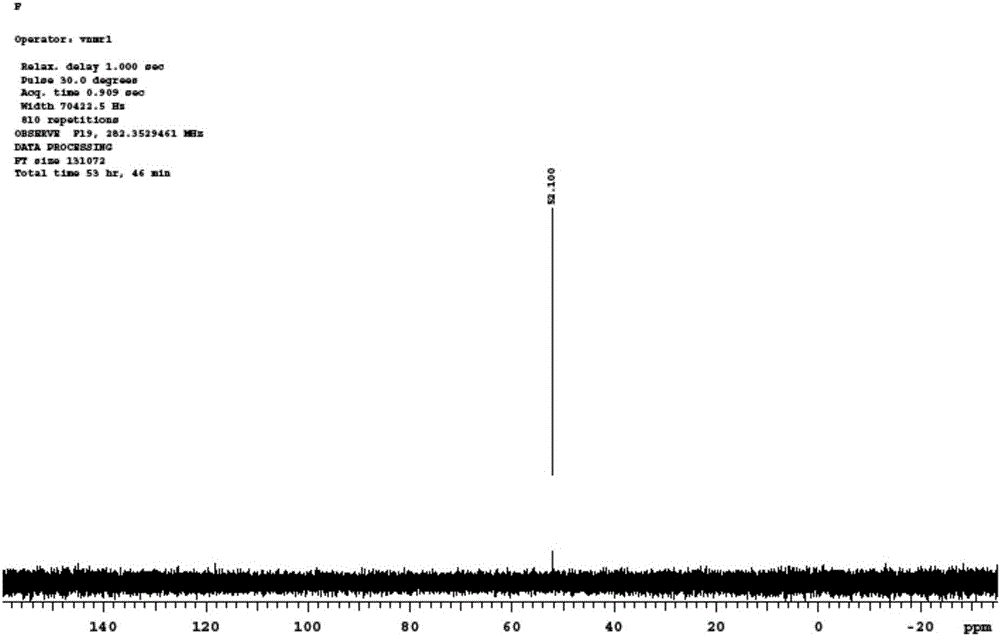
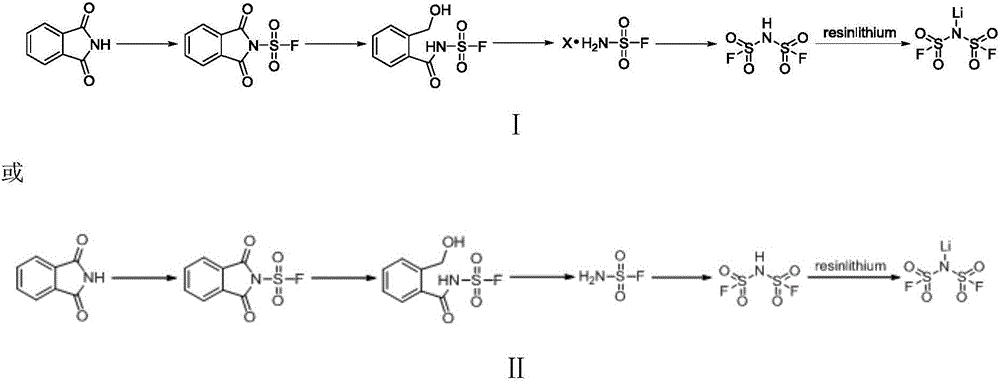
Method for preparing imidodisulfuryl fluoride lithium salt by means of phthalimide
A technology of bisfluorosulfonimide lithium salt and phthalophthalosulfonamide is applied in the field of lithium ion battery electrolyte, which can solve the problems of explosion safety, difficulty in setting the exact amount of ammonia, etc., and achieves cheap raw materials and high yield , the effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 A kind of preparation method of resin lithium
[0042] 1) Weigh 10g of the resin shown in formula (1), add it into a sealed glass tube, lower the temperature to 5°C, slowly add 20g of concentrated sulfuric acid, and raise the temperature to 30-40°C for 1 hour. After the reaction is complete, After the temperature dropped to 0-5°C, 20g of lithium hydroxide was added to carry out neutralization reaction. After the band reaction was completed, it was filtered, washed with ethanol or methanol three times, dried, and 13.8g of lithium resin was collected, and the yield was not counted.
[0043]
[0044] The specific reaction formula involved in the above-mentioned preparation method is as follows:
[0045]
Embodiment 2
[0047] Weigh 10g of the resin shown in formula (2), add it into a sealed glass tube, lower the temperature to 5°C, add 20g of concentrated sulfuric acid slowly, raise the temperature to 30-40°C and react for 1 hour, after the reaction is completed, the temperature will drop After reaching 0-5°C, 10 g of lithium carbonate was added to carry out neutralization reaction. After the band reaction was completed, filter, wash with ethanol or methanol three times, dry, and collect 13.1 g of resin lithium salt, the yield was not counted.
[0048]
[0049] The specific reaction formula involved in the above-mentioned preparation method is as follows:
[0050]
Embodiment 3
[0052] Weigh 14.7g of phthalimine and 19.5mL of triethylamine, dissolve it in 150mL of dichloromethane, add it into a three-necked flask, and slowly add 8.8mL of chlorofluorosulfonyl dropwise after the temperature drops to -20°C. For 20 minutes, the temperature of the entire dropwise addition reaction was kept at -20°C to -10°C. After the dropwise addition was completed, the temperature was raised to 35°C, and the reaction was continued for 12 hours. After the reaction was completed, 200 mL of distilled water was added for extraction. After collecting the organic phase, pour MgSO4 was added to dry it, filtered, and the organic solvent was distilled off under reduced pressure to obtain 22 g of phthalofluorosulfonamide, and the yield (calculated as phthalimine) was 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com