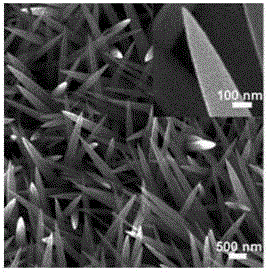
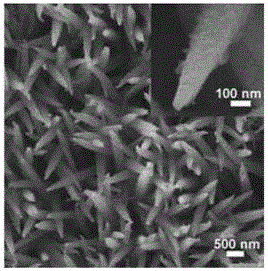
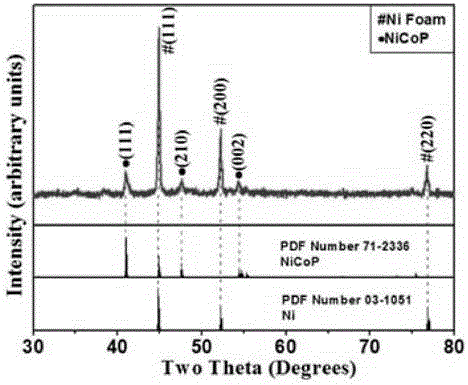
Preparation method for NiCoP nanowire electro-catalytic electrode
A technology of catalytic electrode and rice wire electricity, applied in the direction of electrode, nanotechnology, nanotechnology, etc., can solve the problems of small specific surface area of coating, high product toxicity, easy pollution of the environment, etc., meet the requirements of conventional equipment, improve catalytic activity, and rich raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) remove the nickel foam surface oxide layer, first prepare 120ml deionized water and 3ml 36.8-38wt% concentrated hydrochloric acid solution, then soak the foam nickel sheet of 4cm * 7cm size in the above solution, after 20 minutes, take out the foam nickel The sheet was washed with ethanol and deionized water three times in sequence, and finally dried in an oven at 60°C for 4 hours to obtain a foamed nickel sheet without an oxide layer on the surface;
[0030] (2) Hydrothermal growth of nickel-cobalt salt compound precursor, first dissolve 2mmol nickel chloride and 4mmol cobalt chloride powder in 60mL deionized water, add 12mmol urea until completely dissolved, and then fix the treated foam nickel sheet vertically In the polytetrafluoroethylene liner (100mL) containing the above solution, after sealing with an autoclave, place it in an oven at 120°C for 8 hours, and finally wash and dry the reaction product to obtain the nickel-cobalt salt compound precursor;
[0031...
Embodiment 2
[0033](1) Remove the nickel foam surface oxide layer, first prepare 150ml deionized water and 5ml 36.8-38wt% concentrated hydrochloric acid solution, then soak the foam nickel sheet of 4cm * 7cm size in the above solution, after 18 minutes, take out the foam nickel The sheet was washed with ethanol and deionized water four times in sequence, and finally dried in an oven at 70°C for 4 hours to obtain a nickel foam sheet without an oxide layer on the surface.
[0034] (2) Hydrothermal growth of nickel-cobalt salt compound precursor, first dissolve 3mol nickel chloride and 6mol cobalt chloride powder in 60mL deionized water, add 18mmol urea until completely dissolved, and then fix the treated foam nickel sheet vertically In a polytetrafluoroethylene liner (100mL) filled with the above solution, seal it with an autoclave, place it in an oven at 120°C for 8 hours, and finally wash and dry the reaction product to obtain the nickel-cobalt salt compound precursor.
[0035] (3) Phospha...
Embodiment 3
[0037] (1) Remove the surface oxide layer of foamed nickel, first prepare 200ml deionized water and 5ml 36.8-38wt% concentrated hydrochloric acid solution, then soak the foamed nickel sheet of 4cm * 7cm size in the above solution, after 22 minutes, take out the foamed nickel The sheet was washed with ethanol and deionized water five times in sequence, and finally dried in an oven at 80°C for 3 hours to obtain a nickel foam sheet without an oxide layer on the surface.
[0038] (2) Hydrothermal growth of nickel-cobalt salt compound precursor, first dissolve 3mol nickel chloride and 6mol cobalt chloride powder in 60mL deionized water, add 18mmol urea until completely dissolved, and then fix the treated foam nickel sheet vertically In a polytetrafluoroethylene liner (100mL) filled with the above solution, seal it with an autoclave, place it in an oven at 120°C for 8 hours, and finally wash and dry the reaction product to obtain the nickel-cobalt salt compound precursor.
[0039] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com