A double center boron fluoride complexed dipyrromethene derivative containing carbazole and bridging group in the middle position and its preparation method
A technology of dipyrromethene and bridging group, which is applied in the field of double-center boron fluoride complexed dipyrromethene derivatives and its preparation, can solve the problem of little change in molecular spectral properties, and achieve high comprehensive yield and synthesis low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
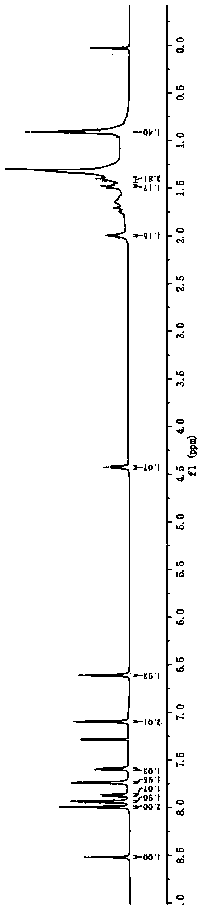
[0037] Synthesis of BDP1.
[0038] (1) Synthesis of intermediate 1 (3,6-dibromocarbazole).
[0039] Add 4 g (24.0 mmol) of carbazole, 8.5 g (48.0 mmol) of N-bromosuccinimide, and 80 mL of anhydrous tetrahydrofuran into a 250 mL single-necked bottle, and heat the reaction system to 80 °C under the protection of argon Stir the reaction for 24 h, stop the reaction, pour the reaction mixture into 200 mL distilled water after cooling to room temperature, extract three times with ethyl acetate, wash the organic layer three times with saturated brine, combine the organic phases, and dry over anhydrous sodium sulfate overnight. The solvent was removed by rotary evaporation, and the residue was subjected to silica gel column chromatography using petroleum ether: ethyl acetate = 30:1 as the eluent to obtain 6.1 g of white solid with a yield of 78%. 1 H NMR (600MHz, CDCl 3 ) δ: 8.13 (s, 2H), 8.10 (s, 1H), 7.52 (d, J = 6.8 Hz, 2H), 7.30 (d, J = 8.6 Hz, 2H). 13 C NMR (151 MHz, CDCl ...
Embodiment 2
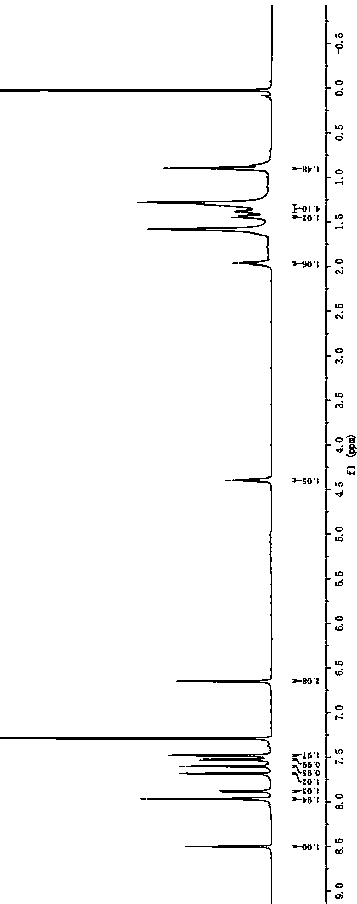
[0051] Synthesis of BDP2.
[0052] (1) Synthesis of intermediate 4b (3,6-bis(5-formylthienyl)-9-octylcarbazole).
[0053] Into a 100 mL three-necked flask, 2 g (3.8 mmol), 1.6 g (8.4 mmol) of 5-bromothiophene-2-carbaldehyde, intermediate 4b was obtained by a method similar to the synthesis of intermediate 4a, an orange-yellow fluorescent solid, and the yield was 68%. 1 H NMR (600 MHz, CDCl 3 ) δ: 8.41 (s, 2H),7.81 (d, J = 8.5 Hz, 2H), 7.77 (d, J = 3.8 Hz, 2H), 7.47 (d, J = 3.8 Hz, 2H), 7.44 (d, J = 8.5 Hz, 2H), 4.31 (t, J = 7.2 Hz, 2H), 1.93–1.84 (m, 2H), 1.39–1.22 (m, 10H), 0.86 (t, J = 6.9 Hz, 2H). 13 C NMR (151 MHz, CDCl 3 ) δ: 182.59, 155.71, 141.57, 137.78, 126.47, 125.16, 124.89, 123.26, 123.15, 118.69, 109.78, 43.54, 31.75, 29.31, 29.13, 22.020, 8.5
[0054] (2) Synthesis of intermediate 5b (3,6-bis[5-(dipyrrolemethyl)thienyl]-9-octylcarbazole).
[0055] Add 0.5 g (1 mmol) of 3,6-bis(5-formylthienyl)-9-octylcarbazole and 10.4 mL (150 mmol) of pyrrole to...
Embodiment 3
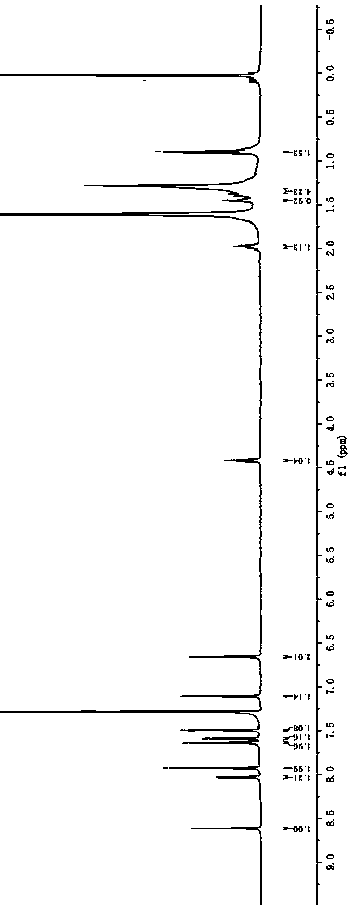
[0059] Synthesis of BDP3.
[0060] (1) Synthesis of intermediate 4c (3,6-bis(5-formylfuryl)-9-octylcarbazole).
[0061] Into a 100 mL three-necked flask, 2 g (3.8 mmol), 1.47 g (8.4 mmol) of 5-bromofuran-2-carbaldehyde, intermediate 4c was obtained by a method similar to that of intermediate 4a, an orange-yellow fluorescent solid, and the yield was 70.7%. 1 H NMR (600 MHz, CDCl 3 ) δ: 9.64 (s,2H), 8.53 (s, 2H), 7.89 (d, J = 8.5 Hz, 2H), 7.37 (t, J = 5.6 Hz, 4H), 6.85(d, J = 3.6 Hz, 2H), 4.23 (t, J = 7.2 Hz, 2H), 1.93–1.74 (m, 2H), 1.36–1.19(m, 10H), 0.86 (t, J = 7.0 Hz, 3H). 13 C NMR (151 MHz, CDCl 3 ) Δ: 176.73, 160.76,151.60, 141.51, 123.84, 123.09, 120.69, 118.03, 109.48, 106.39, 43.40, 31.75,29.29, 29.15, 27.23, 22.59, 14.07. forC 30 h 29 NO 4 : 467.210, found: 467.244 [M] + .
[0062] (2) Synthesis of intermediate 5c (3,6-bis[5-(dipyrrolemethyl)furyl]-9-octylcarbazole).
[0063] Using a method similar to the synthesis of intermediate 5a, intermediate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com