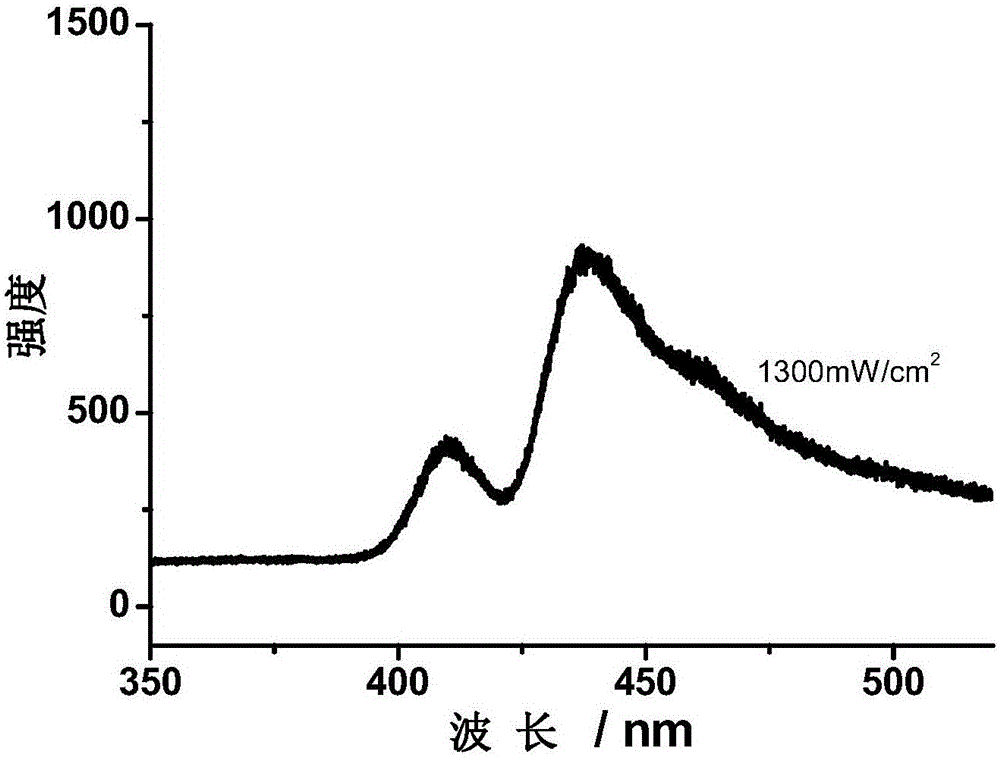
Dendritic polymer structure based triplet-triplet annihilation upconversion luminescent material
A triplet annihilation, luminescent material technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of low doping, inhibition, aggregation and diffusion of photosensitizers and annihilation agents, etc. Good capacitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Doping preparation of triplet-triplet annihilation upconversion luminescent materials based on dendrimer structure:
[0061] 1. Synthesis of an annihilation agent G1-DPA with a dendritic structure of the first generation
[0062] (1) Synthesis of aryl ether dendrimer G1
[0063] Put 5.3g of 3,5-dihydroxybenzyl alcohol, 20g of bromobenzyl bromide, 35g of potassium carbonate and a catalytic amount of 18-C-6 in a 250mL three-necked flask, add about 150mL of dry acetone, and stir and reflux the reaction under a nitrogen atmosphere 10h. Stop the reaction and cool to room temperature, filter under reduced pressure to remove the solid potassium carbonate, and spin the filtrate to remove the solvent under reduced pressure. The spin-dried solid was dissolved in dichloromethane and washed with water to remove 18-C-6. The organic phase was dried with anhydrous magnesium sulfate and filtered, and the filtrate was spin-dried under reduced pressure to obtain a crude product. The c...
Embodiment 2
[0074] Doping preparation of triplet-triplet annihilation upconversion luminescent materials based on dendrimer structure:
[0075] 1. Synthesis of annihilation agent G2-DPA with 2nd generation dendritic structure
[0076] (1) Synthesis of aryl ether dendrimer G2
[0077] Take 1.0 equivalent of G1 prepared in Example 1 and place it in a three-necked flask, dissolve it with a minimum amount of redistilled THF, add 1.25 equivalents of carbon tetrabromide and 1.25 equivalents of triphenylphosphine under a nitrogen atmosphere, and Stir the reaction for 5 minutes, and monitor the progress of the reaction with TLC. If the raw materials are not completely reacted, add the same equivalent of carbon tetrabromide and triphenylphosphine until the raw materials are completely reacted. After the reaction was complete, water was added to the reaction solution to quench the reaction, the water phase was removed by liquid separation, the organic phase was dried with anhydrous magnesium sulfa...
Embodiment 3
[0086] Doping preparation of triplet-triplet annihilation upconversion luminescent materials based on dendrimer structure:
[0087] 1. Synthesis of annihilation agent G3-DPA with 3rd generation dendritic structure
[0088] (1) Synthesis of aryl ether dendrimer G3
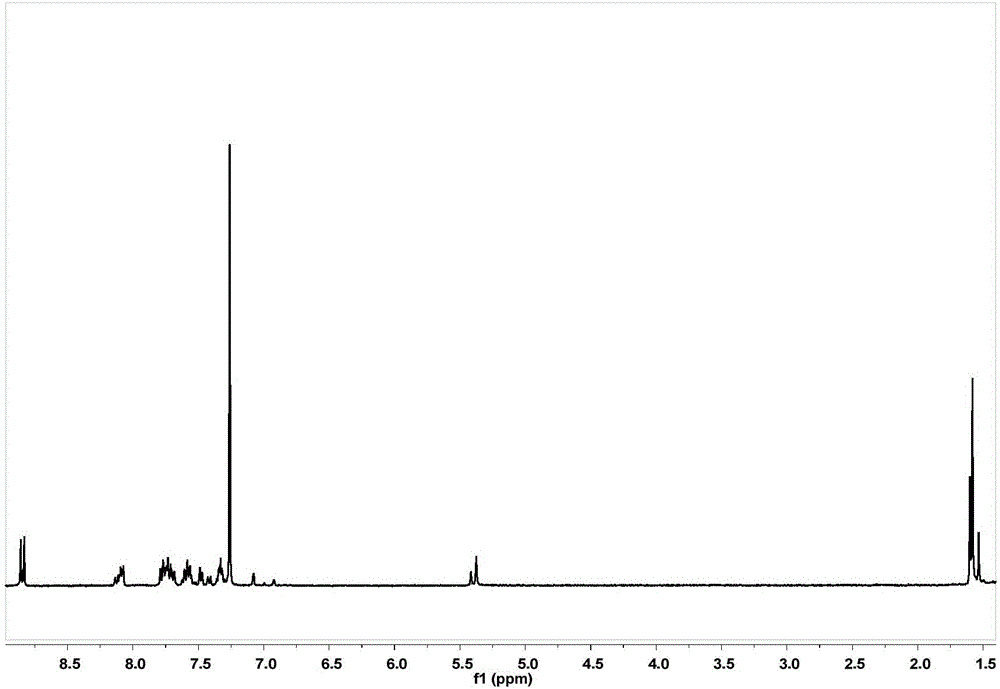
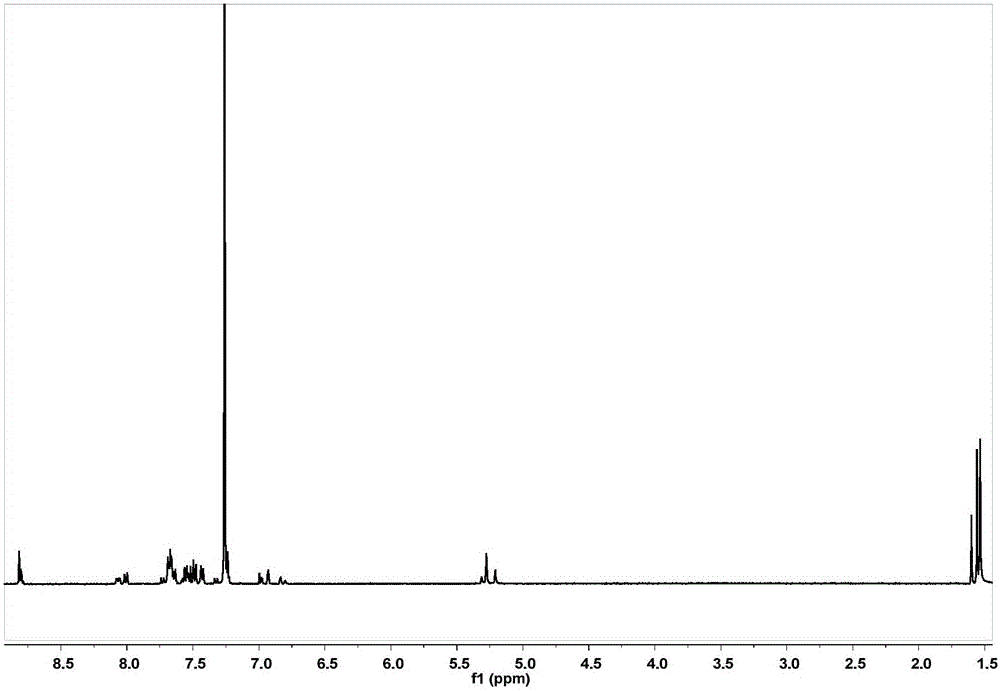
[0089] Get G2 prepared in Example 2, prepare G2-Br according to the method for synthesizing G1-Br in Example 2, and the gained crude product is separated by column chromatography (eluent is dichloromethane / petroleum ether=1 / 1) , the white solid product G2-Br was finally obtained with a yield of 89%. 1 H NMR (400MHz, CDCl 3 ,ppm): δ=7.49(d,J=8.4Hz,8H),7.27(d,J=8.5Hz,8H),6.63(d,J=2.2Hz,4H),6.60(d,J=2.2Hz , 2H), 6.50(t, J=2.2Hz, 2H), 6.47(t, J=2.2Hz, 1H), 4.98(s, 8H), 4.95(s, 4H), 4.40(s, 2H).
[0090] G2-Br and 3,5-dihydroxybenzyl alcohol were prepared according to the method for synthesizing G1 in Example 1 to prepare G3. After the crude product was separated by column chromatography (eluent was dichloromethane),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com