Method for preparing zeolite imidazate framework material
A technology of zeolite imidazolate and framework structure, applied in the directions of cobalt organic compounds, zinc organic compounds, etc., can solve the problems of unsuitable industrial production and no advantages, and achieve the effects of simple operation, energy saving and productivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) 59.4 mg of zinc nitrate hexahydrate (0.2 mmol) was dissolved in 10 ml of ethanol solution, and the concentration was 0.02 mol / liter;
[0023] (2) 32.8 mg of 2-methylimidazole (0.4 mmol) was dissolved in 10 ml of ethanol solution, and the concentration was 0.04 mol / liter;
[0024] (3) Dissolving 48 mg of sodium hydroxide (1.2 mmol) in 1 ml of water, the concentration is 1.2 mol / liter;
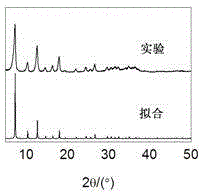
[0025] (4) under normal temperature and pressure stirring, the mol ratio of metal zinc ion, 2-methylimidazole, sodium hydroxide is 1:2:6, by proportioning, the zinc nitrate solution that step (1) is made and step ( 2) Mix the prepared 2-methylimidazole solution, then add the prepared sodium hydroxide solution in step (3), after the addition is completed, continue stirring and reacting for 1 hour at normal temperature and pressure; the resulting mixture is centrifuged, After washing and drying, the zeolite imidazolate skeleton structure material is obtained. Yield 95%. Comparing the...
Embodiment 2
[0027] (1) Dissolve 2970 mg of zinc nitrate hexahydrate (0.01 mole) in 10 milliliters of methanol solution with a concentration of 1 mole / liter;
[0028] (2) 2460 mg of 2-methylimidazole (0.03 moles) was dissolved in 10 milliliters of methanol solution, and the concentration was respectively 3 moles / liter;
[0029] (3) Dissolve 5600 mg of potassium hydroxide (0.1 mole) in 5 milliliters of water at a concentration of 12 moles / liter;
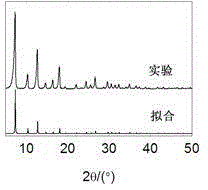
[0030] (4) under normal temperature and pressure stirring, the mol ratio of metallic zinc ion, 2-methylimidazole, potassium hydroxide is 1:3:10, by proportioning, the zinc nitrate solution that step (1) is made and step ( 2) The prepared 2-methylimidazole solution is mixed, then the potassium hydroxide solution prepared in step (3) is added, after the addition is completed, the reaction is continuously stirred at normal temperature and pressure for 2 hours; the resulting mixture is centrifuged, After washing and drying, the zeolite imidazolate sk...
Embodiment 3
[0032] (1) 291 milligrams of cobalt nitrate hexahydrate (1 mmol) are dissolved in 10 milliliters of aqueous solution, and concentration is 0.1 mol / liter;
[0033] (2) 492 mg of 2-methylimidazole (6 mmol) was dissolved in 10 ml of aqueous solution, and the concentration was 0.6 mol / liter;
[0034] (3) The concentration of ammonia water is 15 mol / liter;
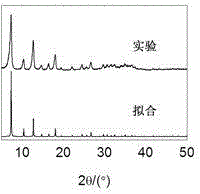
[0035] (4) Under stirring at normal temperature and pressure, the mol ratio of metal ion, imidazole compound, inorganic base is 1:6:18, according to proportioning, the cobalt nitrate solution that step (1) is made and step (2) are made The 2-methylimidazole solution was mixed, and then the ammonia solution of step (3) was added. After the addition was completed, the reaction was continuously stirred at normal temperature and pressure for 0.5 hours; the resulting mixture was centrifuged, washed, and dried to obtain zeolite imidazolate Skeleton material. Yield 85%. Comparing the obtained XRD pattern with the simulated pattern by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com