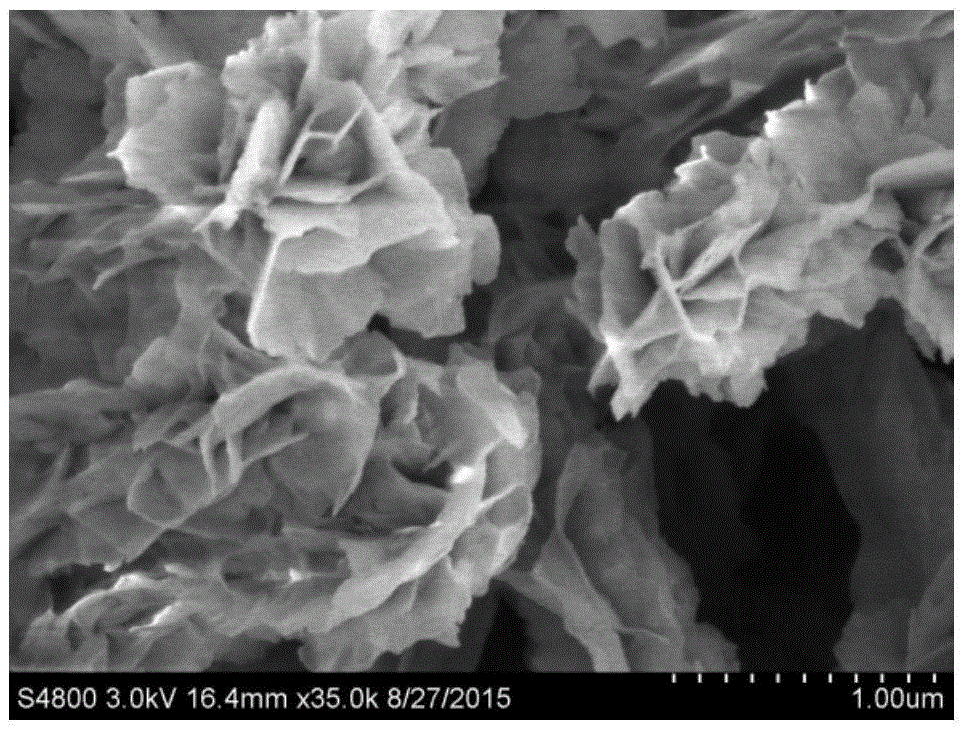
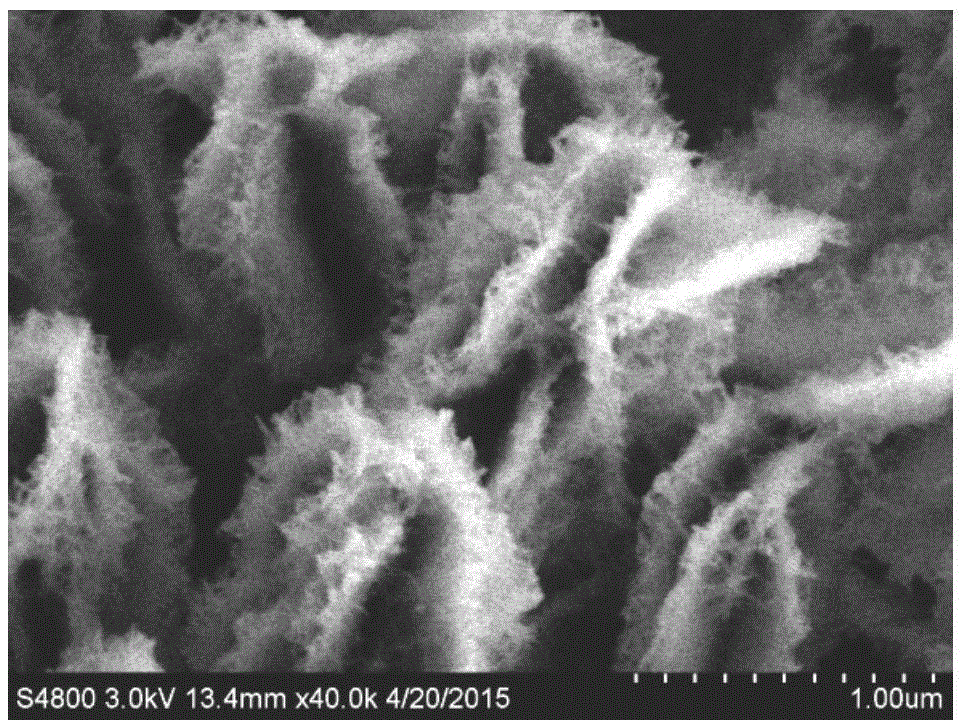
Preparation method for flexible solid supercapacitor Cu(OH)2@Ni2(OH)2CO3 multistage nanoarray electrodes
A supercapacitor and nanoarray technology, which is applied in the manufacture of hybrid/electric double layer capacitors, nanotechnology, nanotechnology, etc., to achieve the effects of economical and environmentally friendly preparation, large specific surface area, and favorable transmission.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The treated foam copper (1×3cm 2 ) in diluted hydrochloric acid, absolute ethanol, and deionized water for 5 minutes, and dried in air. Prepare 2M sodium hydroxide solution and 0.2M ammonium persulfate solution, take 10ml each in a beaker, and stir well. Immerse the washed copper foam into the above mixed solution, shake the beaker in due course, and react at room temperature for 6 minutes. When the surface color of the foamed copper turns into light blue, the sample is taken out, washed alternately with absolute ethanol and deionized water, placed in a vacuum drying oven, and dried at 60°C for 5 hours. Then prepare 2mM nickel nitrate hexahydrate solution and 4mM urea solution, take 10ml of each in a 20ml reaction kettle, and place the above-mentioned dried samples in the mixed solution. Then the reactor was heated to 100°C in an oven, and after 5 hours, the samples (ie, electrodes) were taken out, washed alternately with absolute ethanol and deionized water, placed i...
Embodiment 2
[0049] The treated foam copper (1×3cm 2 ) in diluted hydrochloric acid, absolute ethanol, and deionized water for 5 minutes, and dried in air. Prepare 2M sodium hydroxide solution and 0.2M ammonium persulfate solution, take 10ml each in a beaker, and stir well. Immerse the washed copper foam into the above mixed solution, shake the beaker in due time, and react at room temperature for 9 minutes. When the surface color of the foamed copper turns into light blue, the sample is taken out, washed alternately with absolute ethanol and deionized water, placed in a vacuum drying oven, and dried at 60°C for 5 hours. Then prepare 2mM nickel nitrate hexahydrate solution and 4mM urea solution, take 10ml of each in a 20ml reaction kettle, and place the above-mentioned dried samples in the mixed solution. Then the reactor was heated to 100°C in an oven, and after 5 hours, the samples (ie, electrodes) were taken out, washed alternately with absolute ethanol and deionized water, placed in ...
Embodiment 3
[0051] The treated foam copper (1×3cm 2 ) in diluted hydrochloric acid, absolute ethanol, and deionized water for 5 minutes, and dried in air. Prepare 2M sodium hydroxide solution and 0.2M ammonium persulfate solution, take 10ml each in a beaker, and stir well. Immerse the cleaned copper foam into the above mixed solution, shake the beaker in due course, and react at room temperature for 12 minutes. When the surface color of the foamed copper turns into light blue, the sample is taken out, washed alternately with absolute ethanol and deionized water, placed in a vacuum drying oven, and dried at 60°C for 5 hours. Then prepare 2mM nickel nitrate hexahydrate solution and 4mM urea solution, take 10ml of each in a 20ml reaction kettle, and place the above-mentioned dried samples in the mixed solution. Then the reactor was heated to 100°C in an oven, and after 5 hours, the samples (ie, electrodes) were taken out, washed alternately with absolute ethanol and deionized water, placed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com