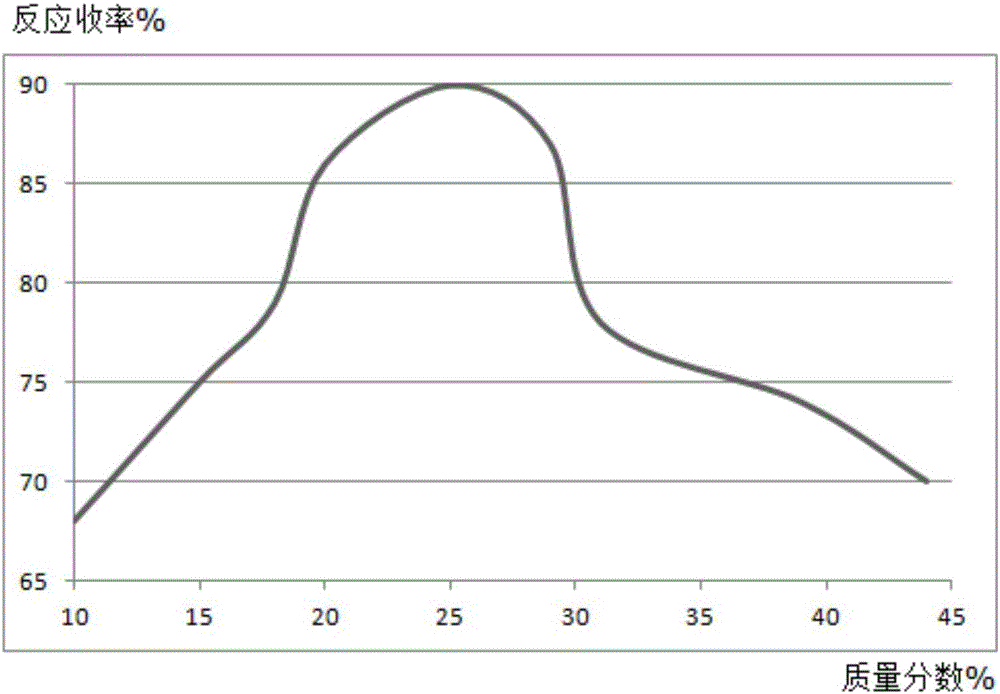
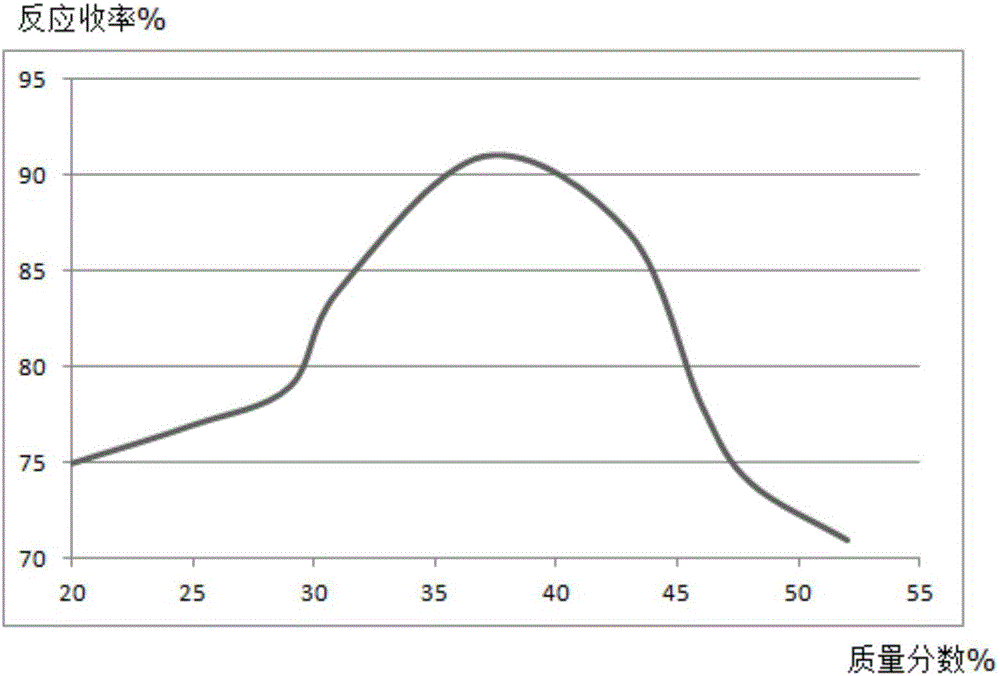
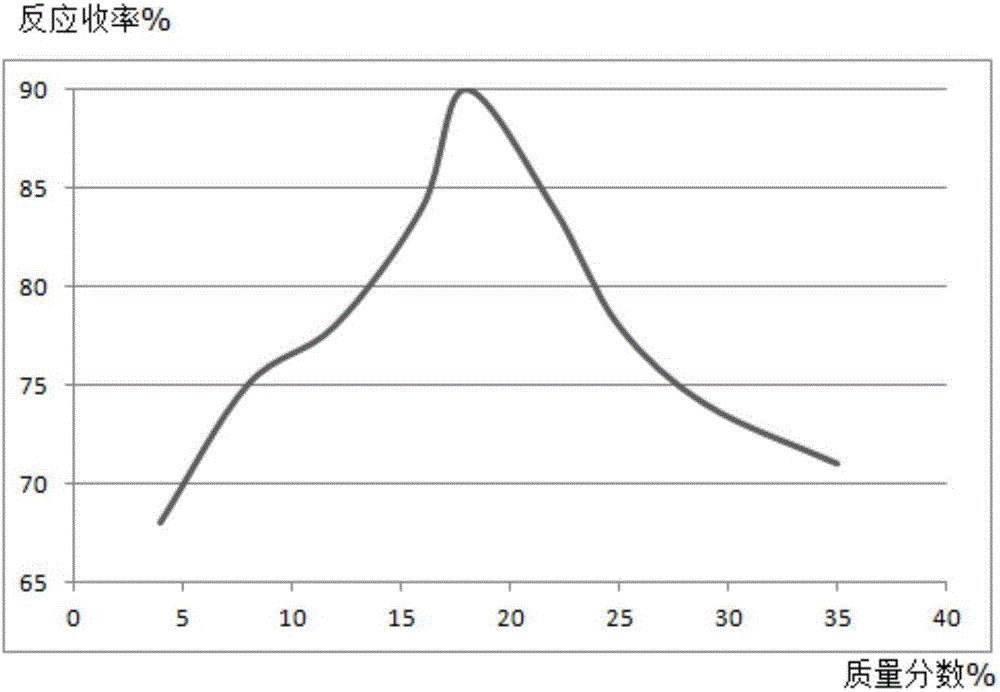
Synthesis method of mesalazine drug intermediate 5-aminosalicylic acid
A technology of aminosalicylic acid and mesalazine, which is applied in the preparation of pharmaceutical intermediates and the synthesis of 5-aminosalicylic acid, a mesalazine pharmaceutical intermediate, can solve complex synthesis methods, many intermediate links in the reaction, and short reaction time. More than 16 hours, etc., to achieve the effect of increasing the reaction yield, increasing the reaction yield, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of synthetic method of mesalazine medicine intermediate 5-aminosalicylic acid, comprises the steps:
[0024] A. In the reaction vessel equipped with a stirrer and a reflux condenser, add 0.78mol of azobenzene salicylate, 230ml of sodium sulfite solution with a mass fraction of 25%, and 2-hydroxyl-5-methyl- 170ml of 3-nitropyridine solution, control the stirring speed at 160rpm, slowly add 0.83mol of molybdenum chloride powder, control the addition time at 3h, raise the solution temperature to 80°C, and react for 7.5h;
[0025] B, adding mass fraction is 210ml of xylene solution of 19%, lowers solution temperature to 16 ℃, separates out solid, is recrystallized in the cyclohexanone solution of 48% in 600ml mass fraction, obtains needle crystal, with 560ml mass fraction Dissolve 24% oxalic acid solution, decolorize with molecular sieve, filter, add 200ml of N-methylformamide solution with a mass fraction of 69% to the filtrate, reduce the temperature of the solutio...
Embodiment 2
[0027] A kind of synthetic method of mesalazine medicine intermediate 5-aminosalicylic acid, comprises the steps:
[0028] A, in the reaction vessel that agitator, reflux condenser are installed, add azobenzene salicylic acid 0.78mol, mass fraction is 25% sodium sulfite solution 220ml, mass fraction is 36% 2-hydroxyl-5-methyl- 150ml of 3-nitropyridine solution, control the stirring speed at 130rpm, slowly add 0.83mol of molybdenum chloride powder, control the addition time at 2h, increase the solution temperature to 79°C, and react for 7h;
[0029] B. Add 190ml of xylene solution with a mass fraction of 19%, lower the temperature of the solution to 13°C, precipitate a solid, and recrystallize in a 48% cyclohexanone solution with a mass fraction of 600ml to obtain needle-like crystals, which are dissolved in 530ml of oxalic acid solution , molecular sieve decolorization, filtering, adding 200ml mass fraction to the filtrate is 68% N-methylformamide solution, reducing the soluti...
Embodiment 3
[0031] A kind of synthetic method of mesalazine medicine intermediate 5-aminosalicylic acid, comprises the steps:
[0032] A. In a reaction vessel equipped with a stirrer and a reflux condenser, add 0.78mol of azobenzene salicylic acid, 200ml of sodium sulfite solution with a mass fraction of 20%, and 2-hydroxyl-5-methyl with a mass fraction of 31%. - 130ml of 3-nitropyridine solution, control the stirring speed at 110rpm, slowly add 0.83mol of molybdenum chloride powder, control the addition time at 2h, raise the solution temperature to 75°C, and react for 6h;
[0033] B, add mass fraction and be 160ml of xylene solution of 162%, reduce solution temperature to 10 ℃, separate out solid, recrystallize in the cyclohexanone solution that is 45% in 600ml mass fraction, obtain needle crystal, use 500ml mass fraction as Dissolve 21% oxalic acid solution, decolorize with molecular sieve, filter, add 200ml of N-methylformamide solution with a mass fraction of 65% to the filtrate, redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com