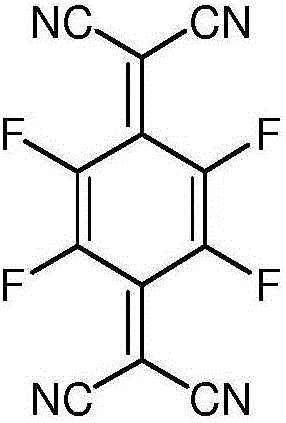
Synthesis method of 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane
A dimethyl pair, synthesis method technology, applied in the field of organic electroluminescent materials, can solve the problems of easy occurrence of danger, increase of risk factor, easy volatilization of bromine, etc., and achieve improved safety, low use environment requirements, and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The synthesis method of 2,3,5,6-tetrafluoro-7,7',8,8'-tetracyanodimethyl-p-benzoquinone provided in the examples of the present invention includes:
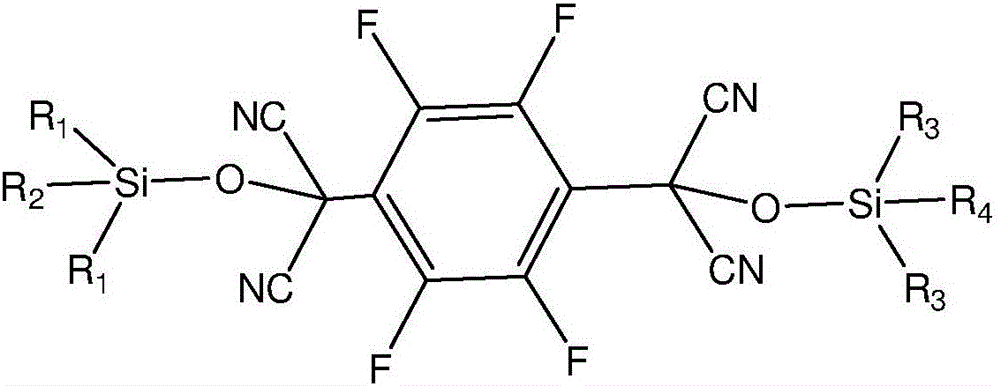
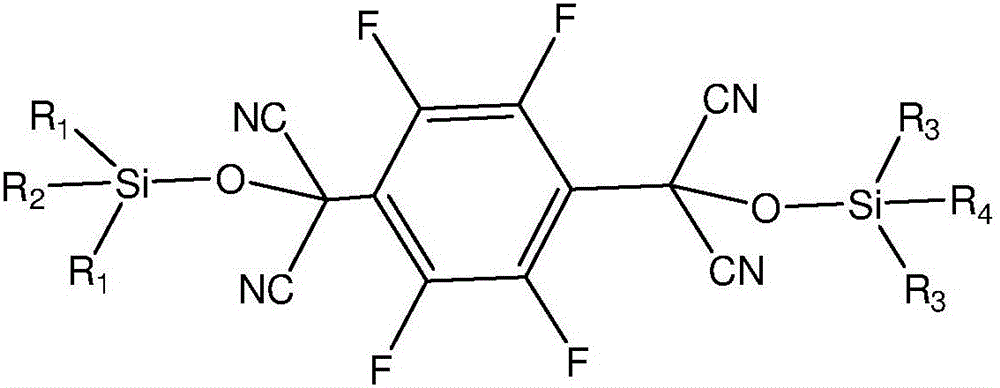
[0041] The first step is to use 2,3,5,6-tetrafluoroterephthaloyl chloride and trialkylsilyl cyanide as raw materials, and use an organic base as a catalyst to carry out condensation reaction under anhydrous and oxygen-free conditions to obtain an intermediate; The chemical structure general formula of described intermediate is:
[0042]
[0043] Among them, R 1 , R 2 , R 3 , R 4 is an alkyl group;
[0044] In the second step, the trialkylsiloxy group in the intermediate is removed to obtain 2,3,5,6-tetrafluoro-7,7',8,8'-tetracyanodimethyl-p-benzoquinone.
[0045] In the synthetic method of 2,3,5,6-tetrafluoro-7,7',8,8'-tetracyanodimethyl-p-benzoquinone provided in the examples of the present invention, 2,3,5,6-tetrafluoro Fluoroterephthaloyl dichloride and trialkylsilyl cyanide are used as raw materials, and the 1...
Embodiment 1
[0081] In the first step, mix 1.42mol of 2,3,5,6-tetrafluoroterephthaloyl chloride, 22.5mol of trimethylsilyl cyanide, and 500mL of pyridine, and heat to 80°C under the protection of nitrogen for condensation Reaction, during the condensation reaction, use thin-layer chromatography to monitor the progress of the reaction. The developer used is dichloromethane. The test results on the silica gel plate are displayed by ultraviolet light. A new product point has been found, which proves that the condensation reaction has reached the end point, and the reaction is ended, and then the pyridine and excess trimethylsilyl cyanide are recovered by distillation under reduced pressure, and the obtained intermediate is put into a desiccator and dried at room temperature for 16 hours, and 1.40mol is collected for drying. later intermediates.
[0082] In the second step, after mixing 1.40 mol of intermediates, 14 mol of phosphorus trichloride and 1500 mL of pyridine, the obtained reaction sys...
Embodiment 2
[0084] In the first step, 1.2mol of 2,3,5,6-tetrafluoroterephthaloyl chloride, 24mol of triethylsilyl cyanide, 150mL of toluene, and 300mL of triethylamine were mixed and heated to Condensation reaction was carried out at 110°C. During the condensation reaction, thin-layer chromatography was used to monitor the progress of the reaction. The developer used was dichloromethane. The test results on the silica gel plate were displayed by ultraviolet light. When stirred at 110°C for 8 hours, the ultraviolet light showed A new product point is generated on the silica gel plate, which proves that the condensation reaction has reached the end point, and the reaction is ended, and then the toluene, triethylamine and excess triethyl cyanosilane are recovered by distillation under reduced pressure, and the obtained intermediate is placed in a desiccator at room temperature After drying for 16 h, 1.15 mol of the dried intermediate was collected.
[0085] In the second step, after mixing 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com