Protease with improved high-temperature activity and thermal stability as well as preparation method and applications of protease
A technology of thermostability and protease, applied in the fields of genetic engineering and enzyme engineering, can solve problems such as environmental pollution, affecting keratin utilization, destroying amino acids, etc., and achieves wide application prospects, improved tolerance, high temperature activity and thermal stability sex-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 protease Pfsp gene synthesis, expression vector construction and mutant construction
[0032] (1) Mutation site analysis and method
[0033] SWISS-MODEL software was used to simulate protease Pfsp, and the tertiary structure of protease Pfsp was obtained; through BLASTP search and alignment, proteins highly similar to protease Pfsp in amino acid sequence were found; ClustalW2 program was used to compare the sequences of these proteins; By analyzing the amino acid sequence and spatial structure of the protease Pfsp, the amino acid sites to be mutated were determined, which were glutamic acid at position 113, alanine at position 189, aspartic acid at position 456, and aspartate at position 458. amino acid and aspartic acid at position 470; the amino acid sequence of protease Pfsp is shown in SEQ ID NO:3.
[0034] (2) Gene optimization synthesis
[0035] The modified and designed gene sequence was synthesized by chemical total synthesis.
[0036] (3) Constr...
Embodiment 2
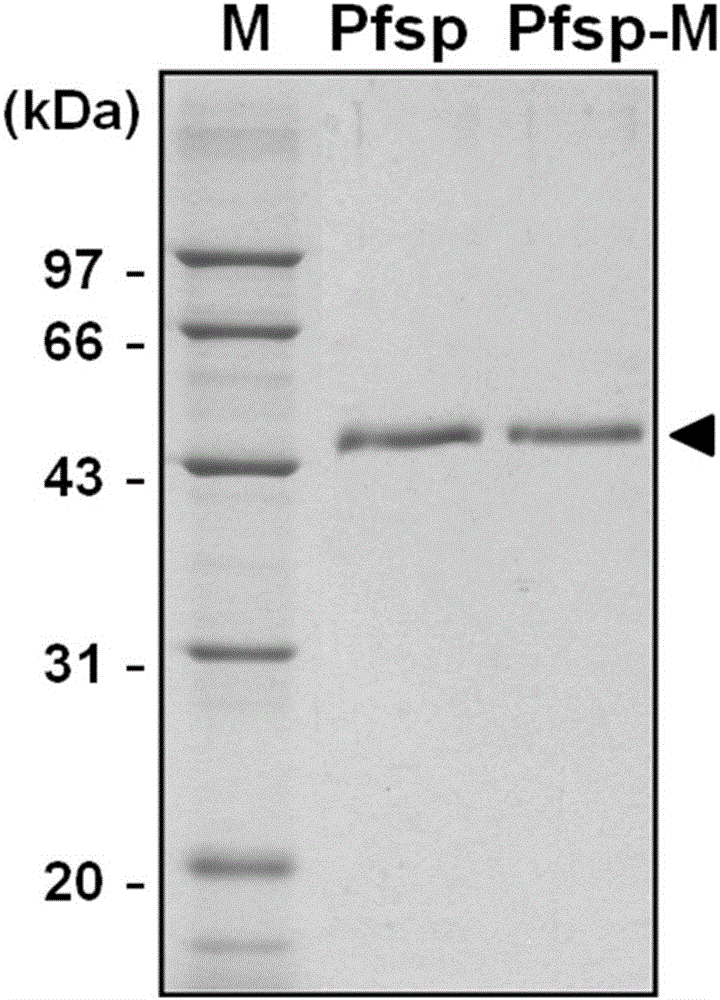
[0046] Expression and purification of embodiment 2 protease Pfsp and protease mutant Pfsp-m in Escherichia coli
[0047] The above two expression vectors pET-26b(+)-Pfsp and pET-26b(+)-Pfsp-m were transformed into Escherichia coli BL21-CodonPlus(DE3)-RIL by heat shock, and the recombinant genes containing the original gene and the mutant gene were respectively obtained. strain.
[0048] Take the Escherichia coli BL21-CodonPlus(DE3)-RIL strain containing the recombinant plasmid and the Escherichia coli BL21-CodonPlus(DE3)-RIL strain containing pET-26b(+) (as a control), and inoculate them respectively in the Mycin in 5 mL LB liquid medium, cultured overnight at 37°C with rapid shaking. Transfer the overnight culture to 50 mL LB liquid medium containing 50 μg / mL kanamycin with 1% inoculum amount, and culture it with rapid shaking at 37 °C until the bacterial liquid OD 600nm Reached around 0.4. Add IPTG to a final concentration of 0.25mM, continue to culture at 16°C for 20h, a...
Embodiment 3
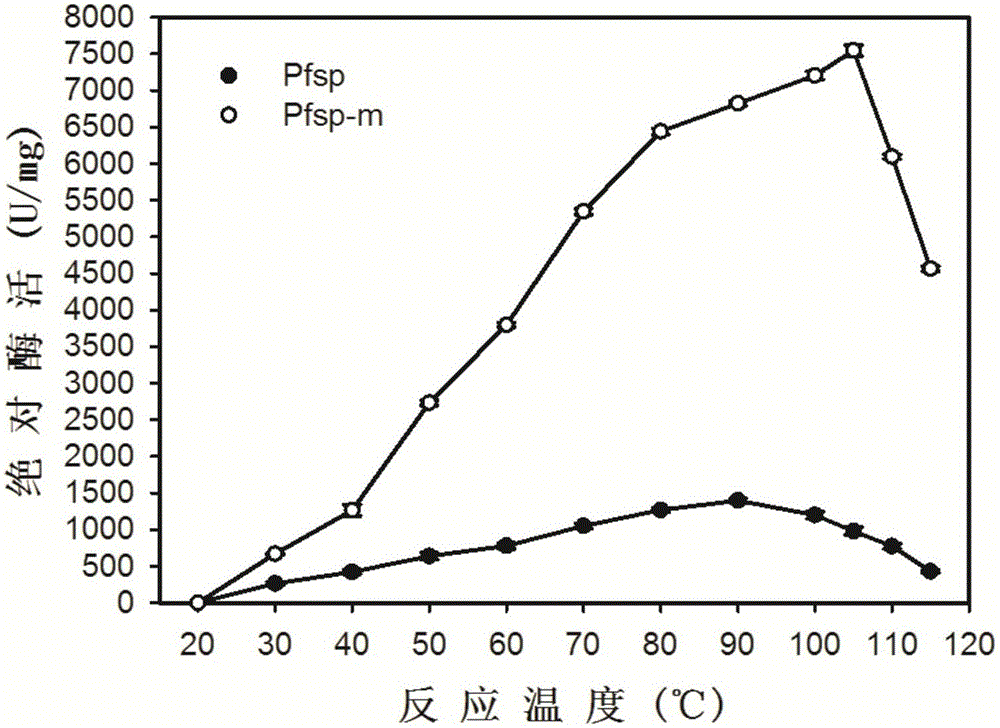
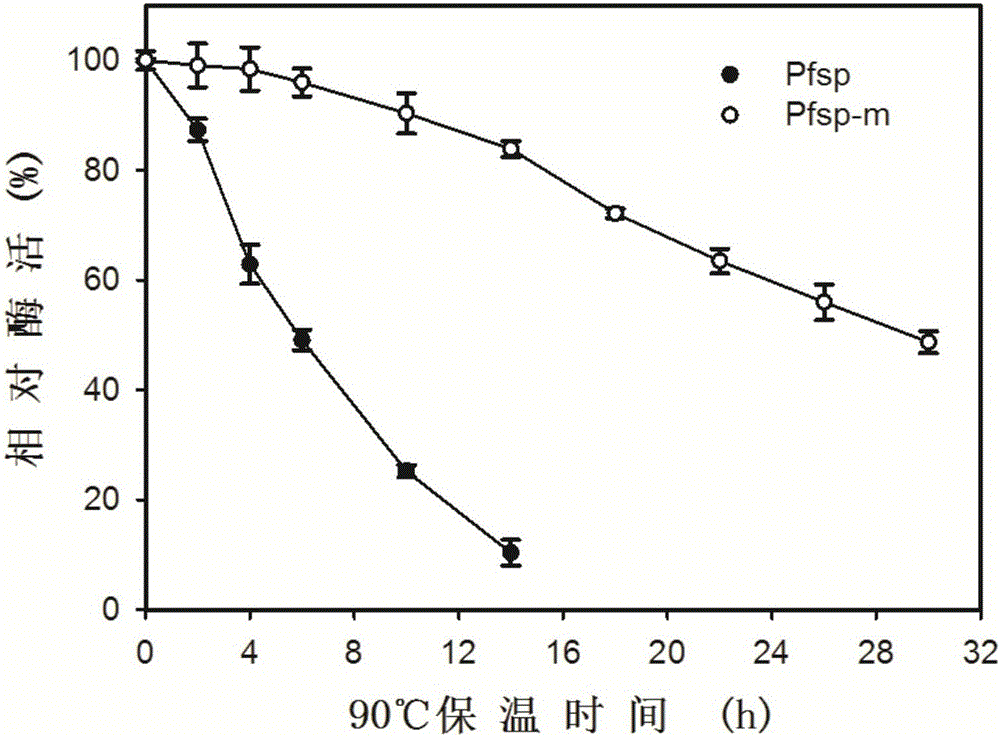
[0051] Partial property analysis of embodiment 3 protease Pfsp and protease mutant Pfsp-m
[0052] (1) Protease activity assay method
[0053] Protease activity was measured using casein as substrate. The reaction was carried out according to the reaction system shown in the table below.
[0054]
[0055] After the reaction was completed, the sample was taken out from the 90°C water bath, immediately placed in an ice-water mixture to cool rapidly, and then 40% TCA was added to terminate the reaction. After standing at room temperature for 15 minutes, centrifuge at 12,000 r / min for 10 minutes, transfer 200 μL of supernatant to a new 1.5 mL Eppendorf tube, and then add 1 mL of Folinol reagent and 200 μL of 0.5M Na 2 CO 3 After developing the color at 40°C for 20 minutes, measure the A of the sample 660nm . According to the standard curve of tyrosine, the amount of enzyme needed to release 1 μg of tyrosine per minute in the above reaction system is defined as one enzyme a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com