Preparation method of biochar-based solid acid and application of biochar-based solid acid to cellulose hydrolysis reaction
A hydrolysis reaction and solid acid technology, applied in the field of biomass resource utilization, can solve the problems of high and difficult batch production of equipment, secondary pollution of sulfuric acid, etc., and achieve the effects of low requirements for reaction equipment, low price and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
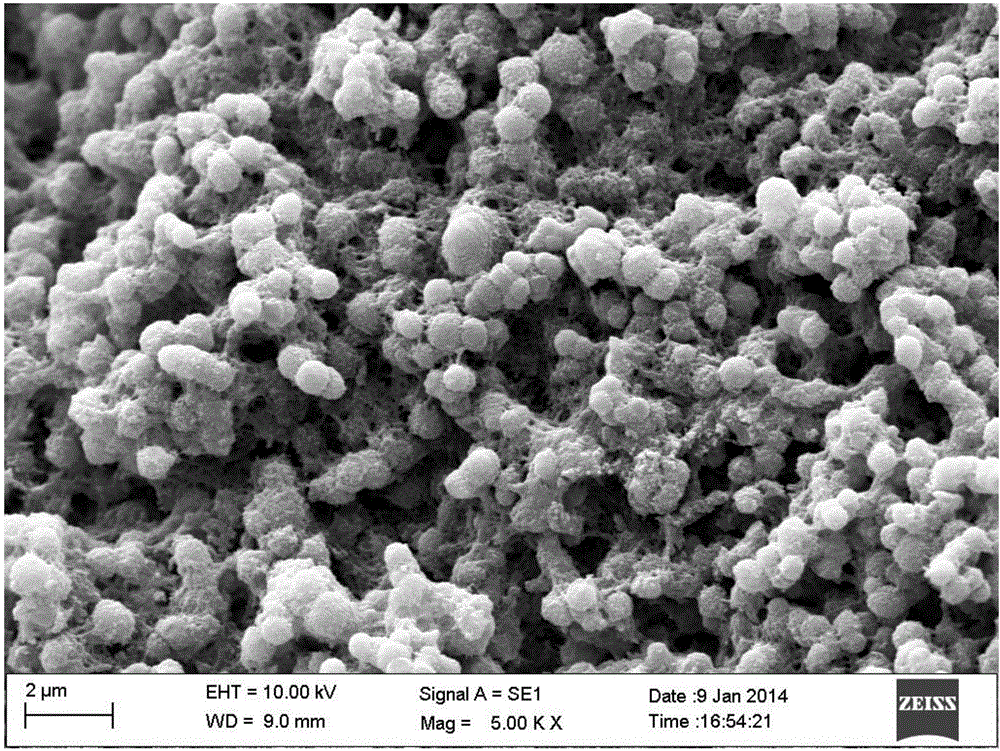
[0017] First weigh 1g of cellulose and 10g of 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), mix them thoroughly in a three-necked glass flask, and then fix the flask at 120°C in an oil bath with magnetic stirring middle. Stir for 60 minutes, after the cellulose is completely dissolved, raise the temperature to 200°C, add 5g of 4-chlorobenzenesulfonic acid dropwise to carry out the synchronous "carbonization-nucleophilic substitution" reaction for 5 hours, after the reaction, the obtained black solid powder is mixed with water and acetone respectively Alternately washed 5 times each until the pH>6, freeze-dried and then heat-dried to obtain a bio-carbon-based solid catalyst with a yield of 49.3%. The SEM spectrum of the catalyst is attached figure 1 As shown, the BET test results prove that its specific surface area is 350m 2 / g, FT-IR shows that the catalyst has -OH, -COOH and -SO 3 H, the elemental analysis catalyst sulfonic acid group content is 4.6mmol / g.
[0018] In...
Embodiment 2
[0020] First, weigh 1 g of glucose and 10 g of [BMIM]Cl, mix them thoroughly in a three-neck glass flask, and then fix the flask in an oil bath with magnetic stirring at 100°C. Stir for 30 minutes, after the glucose is completely dissolved, raise the temperature to 150°C, add 4.17g of 3-fluoromethanesulfonic acid dropwise to carry out the synchronous "carbonization-nucleophilic substitution" reaction for 5 hours, after the reaction, the obtained black solid powder is mixed with water and acetone respectively Alternately washing each 5 times until pH > 6, freeze-drying and then heat-drying to obtain a biocarbon-based solid acid with a yield of 66.9%. The catalyst BET test results prove that its specific surface area is 420m 2 / g, FT-IR shows that the catalyst has -OH, -COOH and -SO 3 H, elemental analysis catalyst sulfonic acid group content is 4.85mmol / g.
[0021] In a 10ml reactor, add 5.0g [BMIM]Cl, 0.25g cellulose and 0.2g deionized water respectively, stir at 100°C for 3...
Embodiment 3
[0023] First weigh 1g of bamboo powder and 10g of [BMIM]Cl, mix them thoroughly in a three-necked glass flask, and then fix the flask in an oil bath at 120°C with magnetic stirring. Stir for 60 minutes, after the bamboo powder is completely dissolved, raise the temperature to 180°C, add 5.35g of sulfamic acid dropwise to carry out the synchronous "carbonization-nucleophilic substitution" reaction for 5 hours, after the reaction, wash the obtained black solid powder alternately with water and acetone 5 times each until the pH>6, freeze-dried and then heat-dried to obtain a biocarbon-based solid acid with a yield of 53.3%. The catalyst BET test results prove that its specific surface area is 120m 2 / g, FT-IR shows that the catalyst has -OH, -COOH, -NH 3 and-SO 3 H, the elemental analysis catalyst sulfonic acid group content is 1.37mmol / g.
[0024] In a 10ml reactor, add 5.0g [BMIM]Cl, 0.25g cellulose and 0.2g deionized water respectively, stir at 100°C for 30min, the cellulos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com