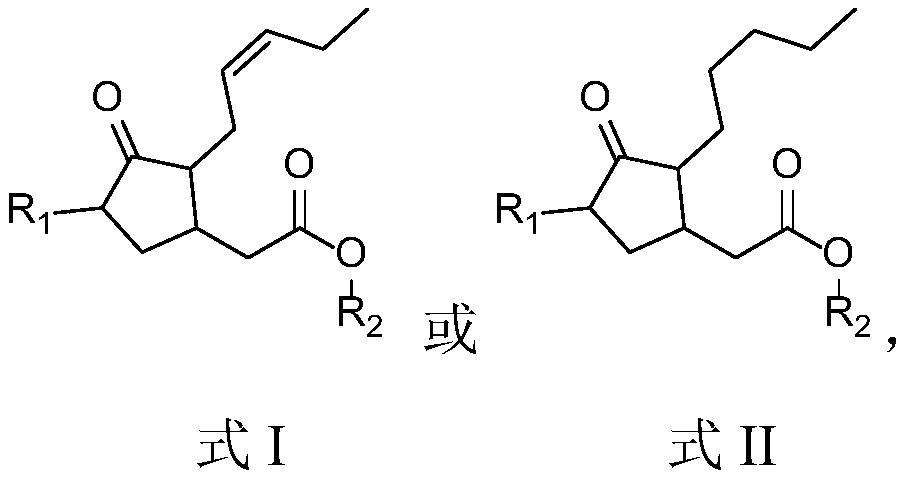
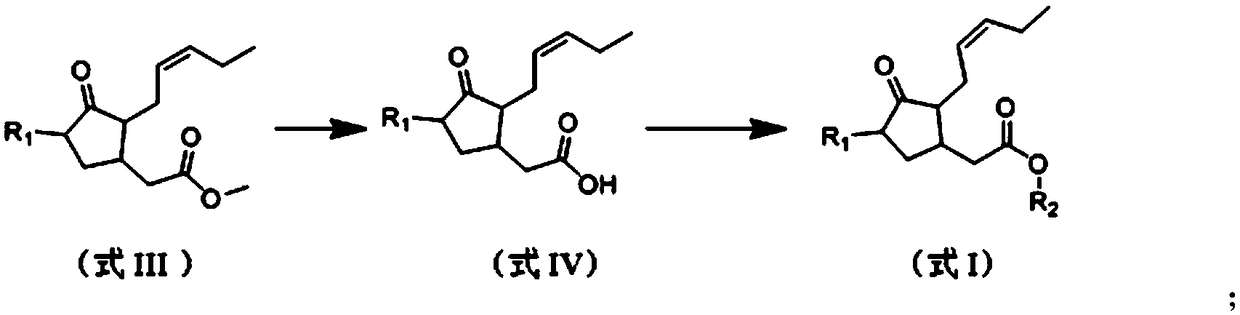
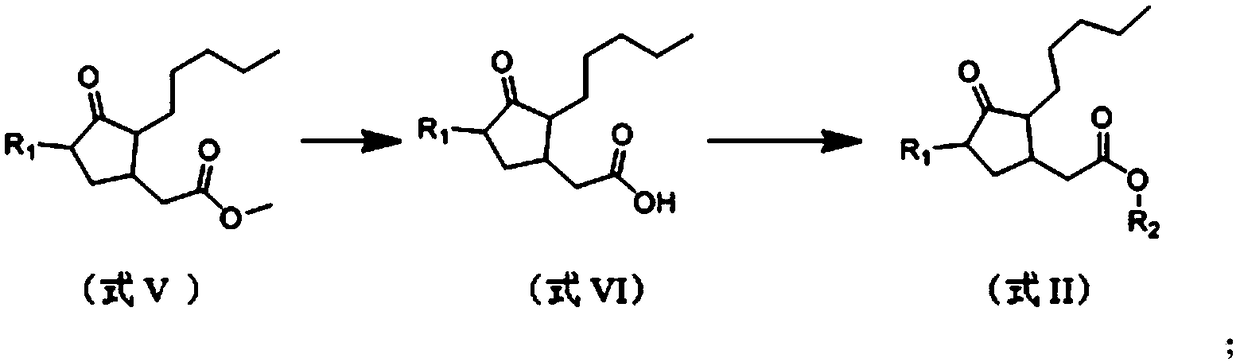
A kind of (trans)-beta-farnesene analog containing jasmonic acid group and its preparation and application
An analog, the technology of jasmonic acid, applied in the application, pest control, pest repellent and other directions, can solve the problems of poor stability, limited application, easy volatility, etc., to achieve improved stability, easy product purification, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of (trans)-β-farnesene analogs containing jasmonic acid groups
[0031] Add 448mg (2mmol) methyl jasmonate (compound shown in formula III) in the mixed solution of 5mL water and 5mL THF, the mixture obtained is added to 168mg (4mmol) LiOH.H 2 O, stirred at room temperature for 1 h, then diluted with 30 mL of water; the aqueous layer was washed successively with 30 mL of hexane, acidified with 1 mol / L hydrochloric acid, and extracted with ethyl acetate; 4 After drying, the solvent was evaporated to give a yellow oily liquid.
[0032] Dissolve the yellow oily liquid into 10mL THF, then add 585mg (3.8mmol) geraniol, DMAP (23mg, 0.2mmol) and DCC (582mg, 2.8mmol) in 5mL THF successively, stir at room temperature for 1.5h, then Diluted with chloroform, filtered off the white solid; the organic phase was washed with 1mol / L hydrochloric acid and acetic acid solution, and then washed with MgSO 4 Drying, evaporation of solvent; Finally, the product obtain...
Embodiment 2
[0055] Example 2: Repellent activity of (trans)-β-farnesene analogues containing jasmonic acid groups against aphids
[0056] More than 20 wingless adult green peach aphids were released from the release port, and humid air passed through activated carbon and distilled water was introduced into each arm through an air pump at 0.2 L / min. The humid air introduced into the test arm passed through 5 μg of the flavor source of (trans)-β-farnesene analogue containing jasmonic acid groups, and the other arm was used as a control arm, and the humid air introduced passed through the solvent. Record the number of aphids in each arm when the sample was introduced for 15 minutes. For each repetition, the olfactometer and leather tube were cleaned with absolute ethanol, the filter paper was replaced, and the two arms were used interchangeably. Each sample experiment was repeated three times. The aphids that crossed the center of the olfactometer by 2cm were included in the treatment group...
Embodiment 3
[0064] Example 3: Aphidicidal activity of (trans)-β-farnesene analogues containing jasmonic acid groups against aphids
[0065] Weigh 50mg of (trans)-beta-farnesene analogs containing jasmonic acid groups in a 20ml weighing bottle with a ten-thousandth balance, and import it into a 10mL volumetric flask to form a 5000mg / L mother liquor; Take 1mL of the mother liquor in a weighing bottle, add 9ml of an aqueous solution containing 0.1% Triton X-100, and mix well to obtain a 500mg / L assay solution. Soybean leaves cultivated indoors that have not been exposed to any pesticides and insects are used to punch out leaves of suitable size with a 15mm diameter puncher, immersed in the diluted measurement solution for 15 seconds, taken out to dry, and placed in the bioassay plate. With the back facing up, 1% agar was added to the bottom to keep it moist, and 20 soybean aphids were inserted into each well, and each was repeated 3 times. Check the results after 48 hours. The criteria for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com