Method for Synthesizing Cationic Polycarboxylate Water Reducer Based on Hoffmann Rearrangement Reaction
A Hoffman rearrangement and cationic technology, which is applied in the field of cationic side chain polycarboxylate superplasticizers, can solve problems such as limiting the application effect of concrete, achieve improved application performance, broad application prospects, and a mild and stable polymerization process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
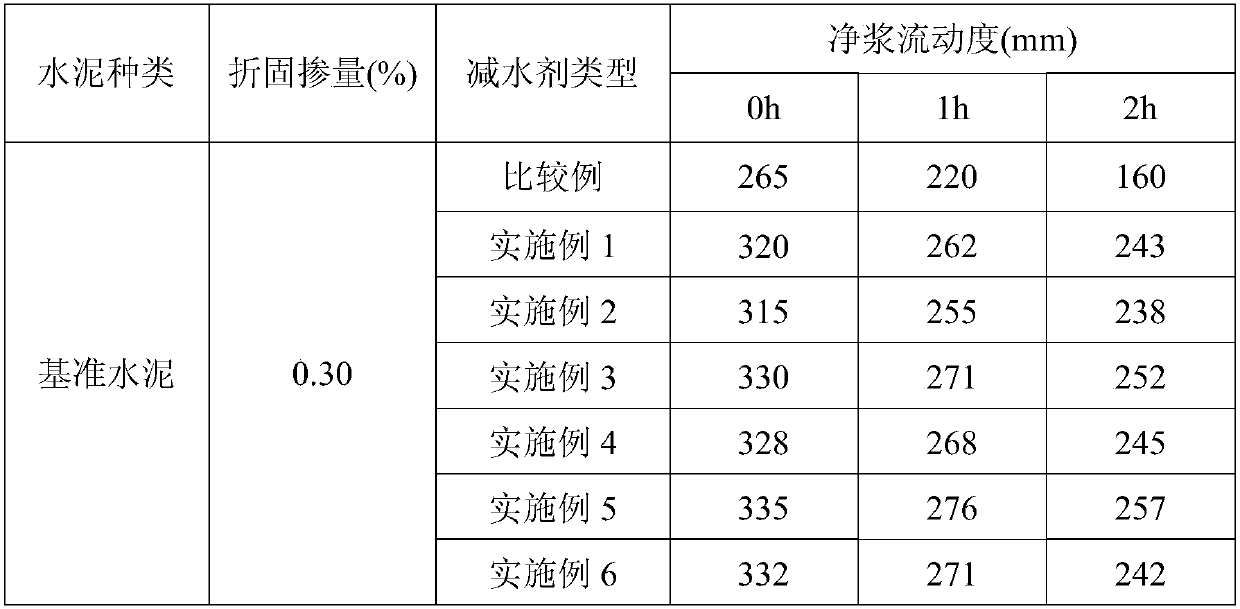
Embodiment 1
[0029] First, add 1.7g of methacrylamide, 2.98g of sodium hypochlorite and 0.8g of sodium hydroxide to the reactor in turn, stir for 8 minutes, and react at a constant temperature of 45°C for 1.5 hours to obtain unsaturated isocyanate; Acryloyloxyethyl dimethyl butyl ammonium bromide and 0.3g of isobutanol were added to another reactor, and then 591.17g of water was added to prepare an aqueous solution with a mass concentration of 2%, and the reactor was filled with nitrogen repeatedly 4 times to remove Seal after 20 minutes of oxygenation, add 2.19g of cerium ammonium nitrate, stir for 18 minutes until it is mixed evenly, continue to heat up to 60°C for polymerization reaction, and react for 6 hours to obtain an aqueous solution of hydroxyl-terminated cation side chains; The chain aqueous solution was vacuumed to remove the water in the system, and the obtained product unsaturated isocyanate, 21.6g polyethylene glycol monomethyl ether (molecular weight = 1800), and 3.37g water...
Embodiment 2
[0031] After the polycarboxylate superplasticizer solution with a mass fraction of 40% obtained in Example 1 was stored at 6° C. for 30 days, its implementation effect was measured.
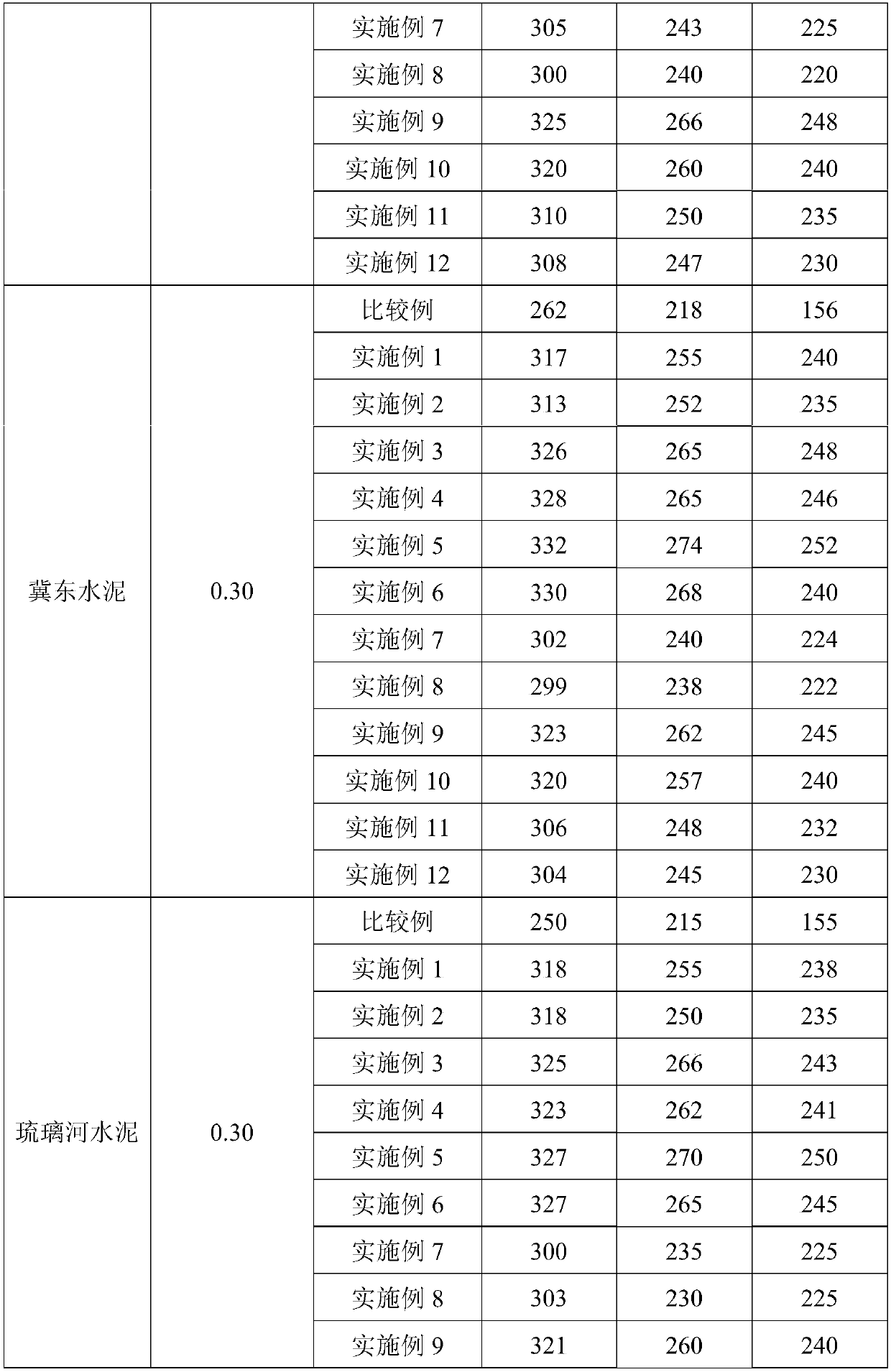
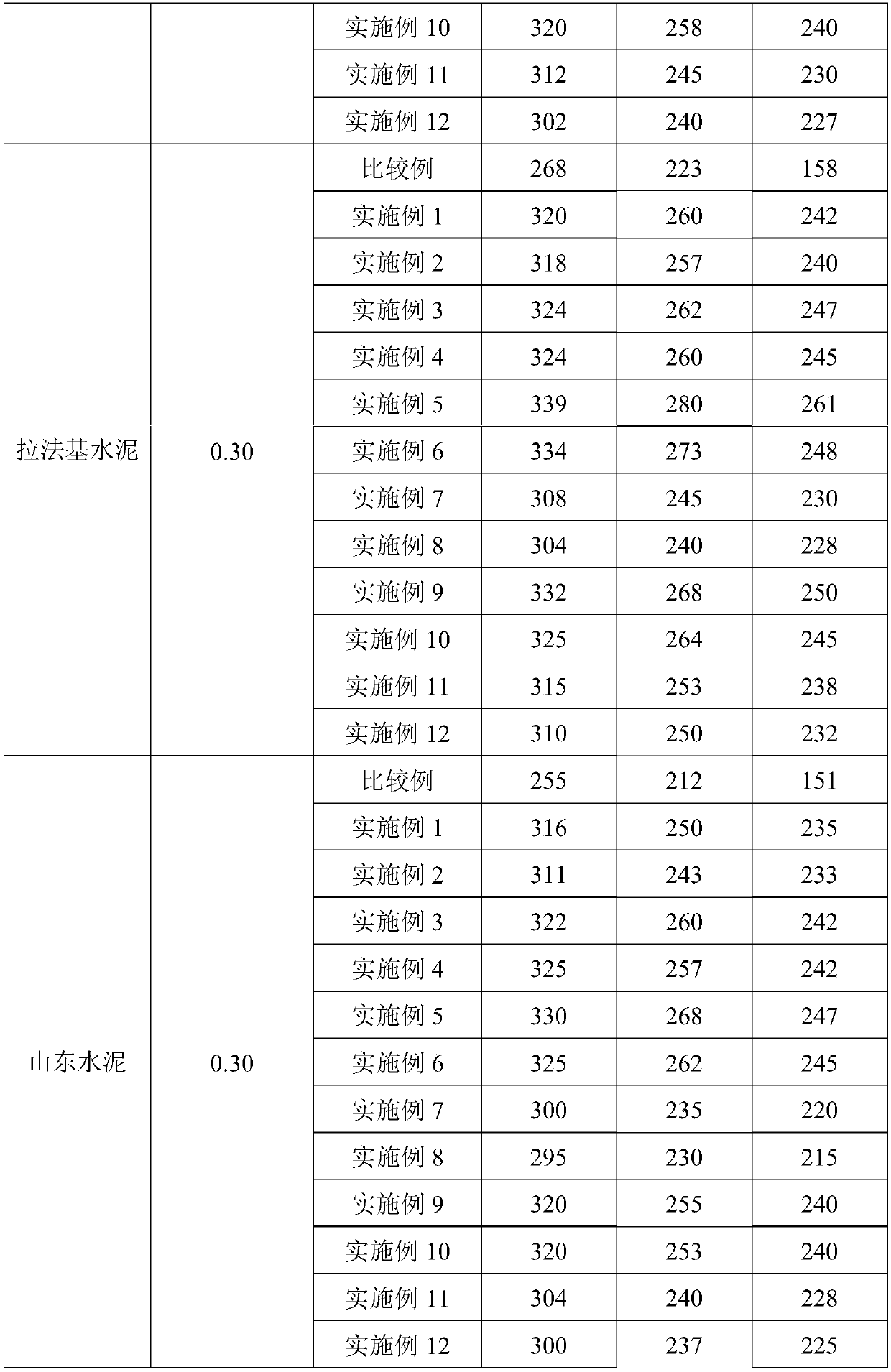
Embodiment 3
[0033] First, add 1.7g of methacrylamide, 3.28g of sodium hypochlorite and 0.8g of sodium hydroxide to the reactor in sequence, stir for 10 minutes, and react at a constant temperature of 35°C for 2.5 hours to obtain unsaturated isocyanate; Methyl allyl ammonium chloride and 0.36g isopropanol are added in another reactor, then add 706.08g water and be mixed with the aqueous solution that mass concentration is 8%, the reactor is filled with nitrogen repeatedly 3 times and deoxygenates after sealing for 30 minutes, Add 1.64g of ammonium cerium nitrate, stir for 10 minutes until it is evenly mixed, continue to heat up to 50°C for polymerization reaction, and react for 8 hours to obtain an aqueous solution of hydroxyl-terminated cation side chains; vacuumize the obtained aqueous solution of hydroxyl-terminated cations and side chains to remove the system Inner water, add the obtained product unsaturated isocyanate, 28g polyethylene glycol monomethyl ether (molecular weight = 2000),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com