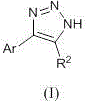
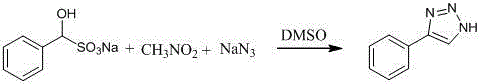Method for preparing 4-aryl-NH-1,2,3-triazole by aid of aldehyde and sodium bisulfite adduct
A technology of aryl and compound, applied in the direction of organic chemistry, can solve problems such as differences in reactivity, achieve simple operation, easy industrial production, and improve production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Synthesis of 4-phenyl-NH-1,2,3-triazole:
[0017] The reaction formula is:
[0018]
[0019] Specific steps: Add 1 mmol of benzaldehyde sodium bisulfite adduct, 2.0 mmol of sodium azide, 4.0 mmol of nitromethane and 1.0 mL of dimethyl sulfoxide into a 10 mL round bottom flask, and heat at 120°C for 4 hours. After the reaction, the reaction system was cooled to room temperature, the reaction solution was all transferred to a separatory funnel, 30 mL of ethyl acetate, 30 mL of water and 10 mL of saturated ammonium chloride aqueous solution were added to extract and separate the liquid, and the organic phase was washed 3 times with water ( 10mL * 3), washed once with saturated brine (10mL), and dried over anhydrous sodium sulfate. The desiccant was filtered off, the filtrate was removed from the solvent under reduced pressure, and the silica gel column chromatography was performed. The eluent was petroleum ether / ethyl acetate (v: v = 4:1). Fractions were collected to ...
Embodiment 2
[0021] Synthesis of 4-phenyl-NH-1,2,3-triazole:
[0022] The reaction formula is:
[0023]
[0024] Specific steps: Add 1.0 mmol benzaldehyde sodium bisulfite adduct, 1.0 mmol sodium azide, 1.0 mmol NaHSO to a 10 mL round bottom flask 3 and 3 mL of dimethyl sulfoxide, the reaction system was replaced with nitrogen, and a N,N dimethylformamide (DMF) solution containing 1.0 mmol of nitromethane was added dropwise at 60°C. After the dropwise addition, continued heating for 10 hours. Other operations are the same as in Example 1, and the yield is 42%.
Embodiment 3
[0026] Synthesis of 4-phenyl-NH-1,2,3-triazole:
[0027] The reaction formula is:
[0028]
[0029] Specific steps: Add 1.0 mmol benzaldehyde, 2.0 mmol sodium azide, 1.0 mmol Na 2 SO 3 and 3 mL of N-methylpyrrolidone (NMP), add dropwise an NMP solution (2 mL) containing 4.0 mmol of nitromethane at 120°C, and continue heating for 5 hours after the addition is complete. Other operations are the same as in Example 1, and the yield is 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com