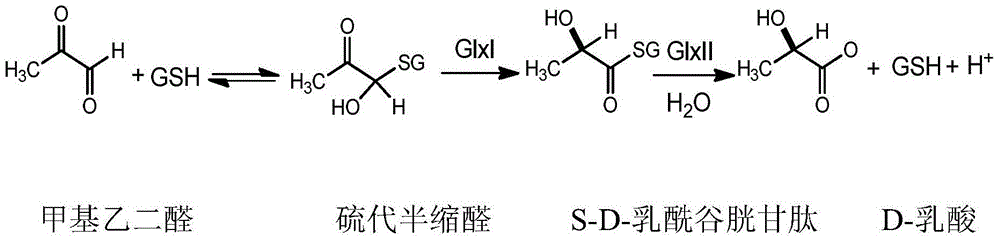
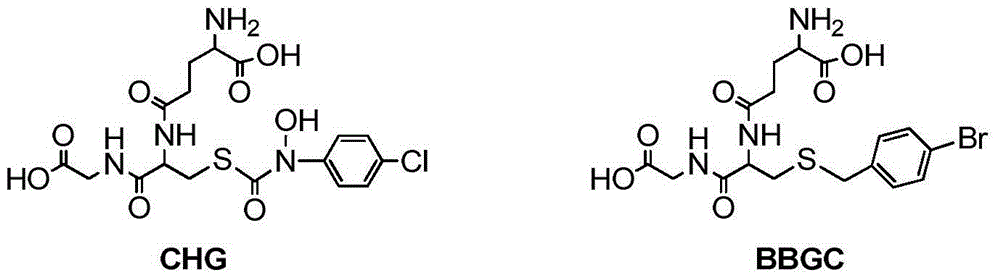
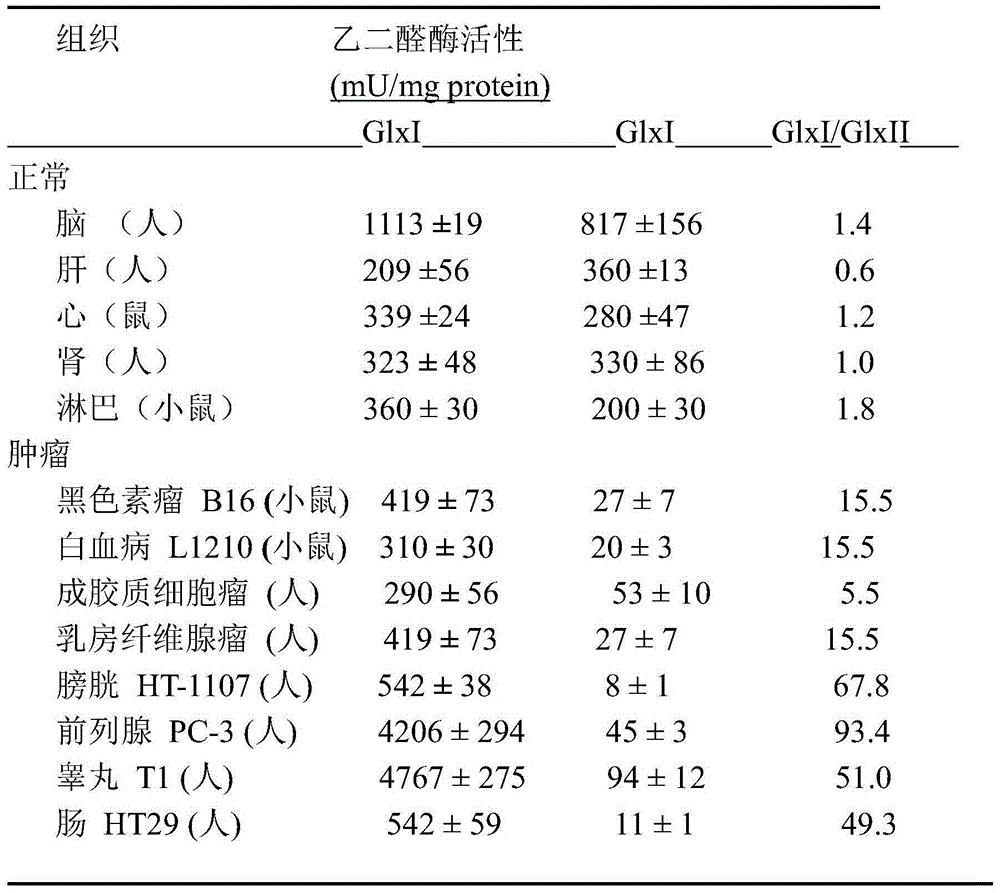
Glyoxalase I irreversible inhibitor and preparation method and application thereof
A technology of dichloromethane and compounds, which is applied in the field of glyoxalase I competitive inhibitors and its preparation, can solve the problems of poor affinity of irreversible inhibitors and lack of breakthroughs in research and development, and achieve good application prospects and drug efficacy Long-lasting, good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The preparation of embodiment 1 compound 15 of the present invention
[0077] The synthetic route is as follows:
[0078]
[0079] The specific synthesis process is as follows:
[0080] Preparation of Compound 2
[0081] Compound 1 (10.00g, 49.02mmol), potassium carbonate (13.53g, 98.04mmol) and potassium hydroxide (2.75g, 49.02mmol) were dissolved in a mixed solvent of THF (90mL) and water (30mL). Stir and add benzyloxycarbonyl succinimide (18.3 g, 73.53 mmol). After the addition, it was naturally raised to room temperature and reacted for 8 hours. Concentrate under reduced pressure to remove THF, extract the aqueous layer with ether, separate the layers, acidify the aqueous layer with citric acid to pH=4, precipitate out, and extract with dichloromethane. The organic layers were combined, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain light yellow oily compound 2 (15.57 g, yield 94%), which was directly used in the next step.
[0082...
Embodiment 2
[0110] Embodiment 2 The preparation of compound 15DE of the present invention
[0111]
[0112] Preparation of compound 16
[0113] Under ice bath, compound 2 (5g, 14.79mmol) and potassium carbonate (3.06g, 22.19mmol) were dissolved in DMF (50mL), magnetically stirred, iodoethane (3.45g, 22.19mmol) was added dropwise to the reaction flask ), after the addition was completed, the reaction was naturally raised to room temperature for 5 h, and the reaction was monitored by TLC to be complete. The reaction solution was added to water (300mL), extracted with ethyl acetate (300mL×2), the organic phases were combined, and the organic phase was washed with saturated aqueous sodium chloride solution (50mL), dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure. After drying the organic solvent, the mixed solvent of ethyl acetate and petroleum ether was stirred to disperse the residue evenly, and then filtered to obtain light yellow solid 16 (4.8 g, yi...
Embodiment 3
[0132] Embodiment 3. Preparation of glyoxalase I inhibitor freeze-dried powder injection of the present invention
[0133] The preparation process of the glyoxalase I inhibitor freeze-dried powder injection of the present invention comprises the following steps:
[0134] 1) Add lyophilized support agent and co-solvent: adjust the pH value of the medicinal solution to 4-9, and add mannitol, a lyophilized support agent with half the weight of the glyoxalase I inhibitor. The co-solvent is β-hydroxymethylcyclodextrin for injection, and the addition amount is equal to that of the glyoxalase I inhibitor.
[0135] The weight ratio of the glyoxalase I inhibitor to the freeze-dried support agent mannitol is 6:1-3:1.
[0136] The lyophilized support agent is selected from one or a mixture of any two of mannitol, xylitol, sorbitol, sodium chloride and dextran; the weight of glyoxalase I inhibitor and β-hydroxymethyl cyclodextrin The ratio is 10:1 to 1:1.
[0137] 2) Depyrogenation: Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com