Preparation method of halogenated polymer
A compound and copolymer technology, applied in the field of preparation of halogenated polymers, can solve the problems of difficult and unsatisfactory preparation of initiator system, and high technical requirements for the preparation of aluminoxane, so as to reduce energy consumption, improve initiation efficiency, Effect of High Polymer Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
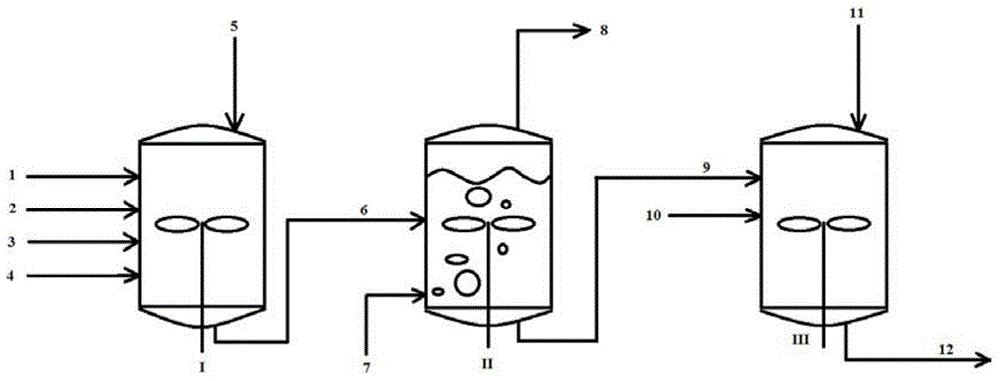
[0034] The invention provides a kind of preparation method of halogenated monoolefin-conjugated diene copolymer, the method comprises the following steps:
[0035] (1) Under cationic polymerization conditions, at least one monoolefin and at least one conjugated diene are contacted with each component in the initiator system in a polymerization solvent to obtain a monoolefin-conjugated diene copolymer containing a solution, the polymerization solvent is composed of at least one first polymerization solvent and at least one second polymerization solvent, the first polymerization solvent is selected from halogenated alkanes, and the second polymerization solvent is selected from alkanes;
[0036] (2) Replace the halogenated alkane in the solution containing the monoolefin-conjugated diene copolymer with at least one replacement solvent and remove unreacted monomers to obtain a replacement solvent containing the monoolefin-conjugated diene copolymer After the solution, the replace...
preparation example 1
[0107] Preparation Example 1: Synthesis of 2-chloro-2,4,4-trimethylpentane (TMPCl)
[0108] A 250 mL round-bottomed three-neck flask was placed in an ice-water bath, and then 30 mL of 2,4,4-trimethyl-1-pentene and 30 mL of dichloromethane were added. Under the condition of continuously feeding dry hydrogen chloride gas, the reaction was carried out for 5h. The mixture obtained by the reaction was neutralized with sodium bicarbonate, then anhydrous magnesium sulfate was added to the solution, and then filtered, the liquid mixture was collected, and vacuum distillation was carried out to collect the fraction at 44°C (2.1332kPa) (yield: 70 % by weight, its purity was determined to be 93% by gas chromatography analysis). After characterization, it was confirmed that the fraction was 2-chloro-2,4,4-trimethylpentane. in, 1 H-NMR (δ, ppm): 1.06(-C(CH 3 ) 3 ), 1.67(-C(CH 3 ) 2 Cl), 1.88(-CH 2 -).
preparation example 2
[0109] Preparation Example 2: Synthesis of Dicumyl Chloride
[0110] Put a 500mL three-necked round-bottomed cauldron equipped with bottomed inlet and outlet pipes and a magnetic stirring device in an ice-water bath, and then add 8g of p-dicumyl alcohol and 10g of CaCl 2 and 100 g of dichloromethane. Continuously feed dry hydrogen chloride gas into the three-necked flask, and stir and react for 10 hours. After the reaction was completed, it was filtered to obtain a clear solution. Hydrogen chloride and methylene chloride were removed in vacuo to obtain 9.1 g of colorless needle crystals (yield: 96% by weight). Dissolve the obtained needle-shaped crystals in 50 mL of n-hexane, filter to remove insoluble impurities, cool the remaining liquid phase to -20°C to -30°C, crystallize with stirring, separate the precipitated crystals from the mother liquor, and collect the crystals. The crystal was determined to be p-dicumyl chloride through characterization. in, 1 H-NMR (δ, ppm): ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com