TCR<->(T Cell Receptor)/PD-1<-> double-negative T cell and construction method thereof
A double-negative, PD-1 technology, applied in the field of biomedicine, can solve the problems of increasing patient burden, low activity, and ineffective CART therapy, and achieve the effect of reducing treatment costs, reducing immune damage, and large-scale preparation in advance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
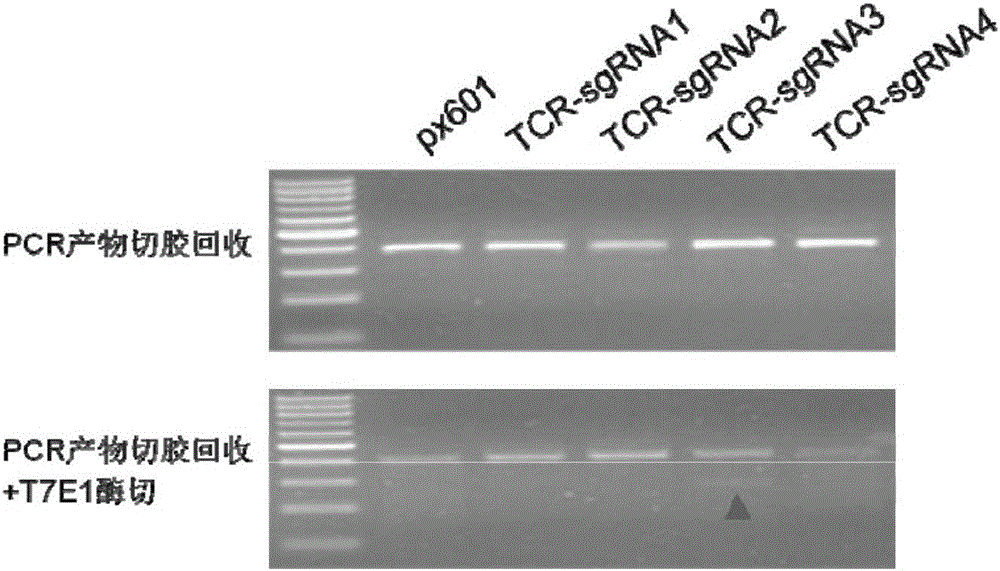
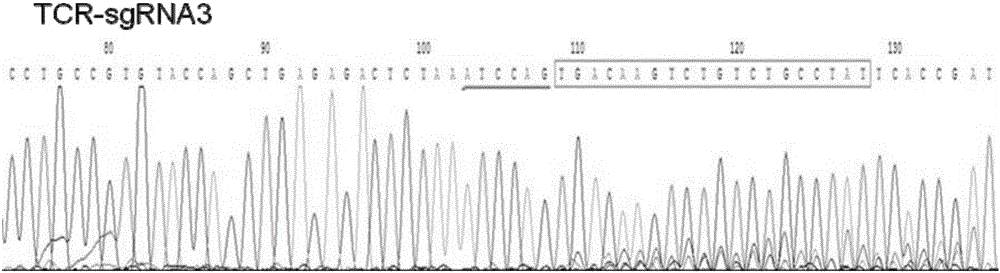
[0066] Example 1 Construction of TCR based on CRISPR / Cas9 system - / PD-1 - sgRNA for double negative T cells
[0067] PD-1 is an inhibitory receptor on the surface of T cells, TCR (T cell receptor) is a molecular structure that T cells specifically recognize and bind to antigen peptide-MHC molecules, usually in the form of complexes with CD3 molecules in T cells Cell surface; the TCR of most T cells consists of alpha and beta peptide chains.
[0068] To build TCR - / PD-1 - Double negative T cells, in order to destroy the reading frame of the target gene as much as possible, the purpose of the present invention is to obtain the target gene TCR-α and PD1 sgRNA with knockout activity. The present invention obtains 15 pairs of targeted genes TCR-α sgRNAs, and 18 targeted genes PD-1 sgRNA.
[0069] Among them, targeting people TCR-α The sgRNA sequence of the gene is selected from any one of SEQ ID NOs: 1 to 15; the reverse complementary DNA sequences of the sequences of...
Embodiment 2
[0072] Example 2 Construction of TCR based on CRISPR / Cas9 system - / PD-1 - DNA oligonucleotides for double negative T cells
[0073] The corresponding DNA oligonucleotides were synthesized according to the sgRNA designed in the above Example 1, CACC was added to the 5' of the forward oligonucleotide, and AAAC was added to the 5' of the reverse oligonucleotide; the sgRNA sequence of the above TCR-α The sequences of the forward oligonucleotides corresponding to SEQ ID NOs: 1 to 4 are respectively SEQ ID NOs: 67, 69, 71 and 73, and the sequences of the corresponding reverse oligonucleotides are respectively SEQ ID NO: 68, 70, 72, 74; the sequences of the forward oligonucleotides corresponding to the sgRNA sequences of PD-1 SEQ ID NOs: 16 to 19 are respectively SEQ ID NOs: 75, 77, 79, 81, and the corresponding reverse oligonucleotides The sequences of the nucleotides are SEQ ID NO: 76, 78, 80, 82, respectively.
[0074] Pair and anneal the above-synthesized forward oligonucleot...
Embodiment 3
[0077] Example 3 Construction method of CRISPR / Cas9-TCR-sgRNA plasmid
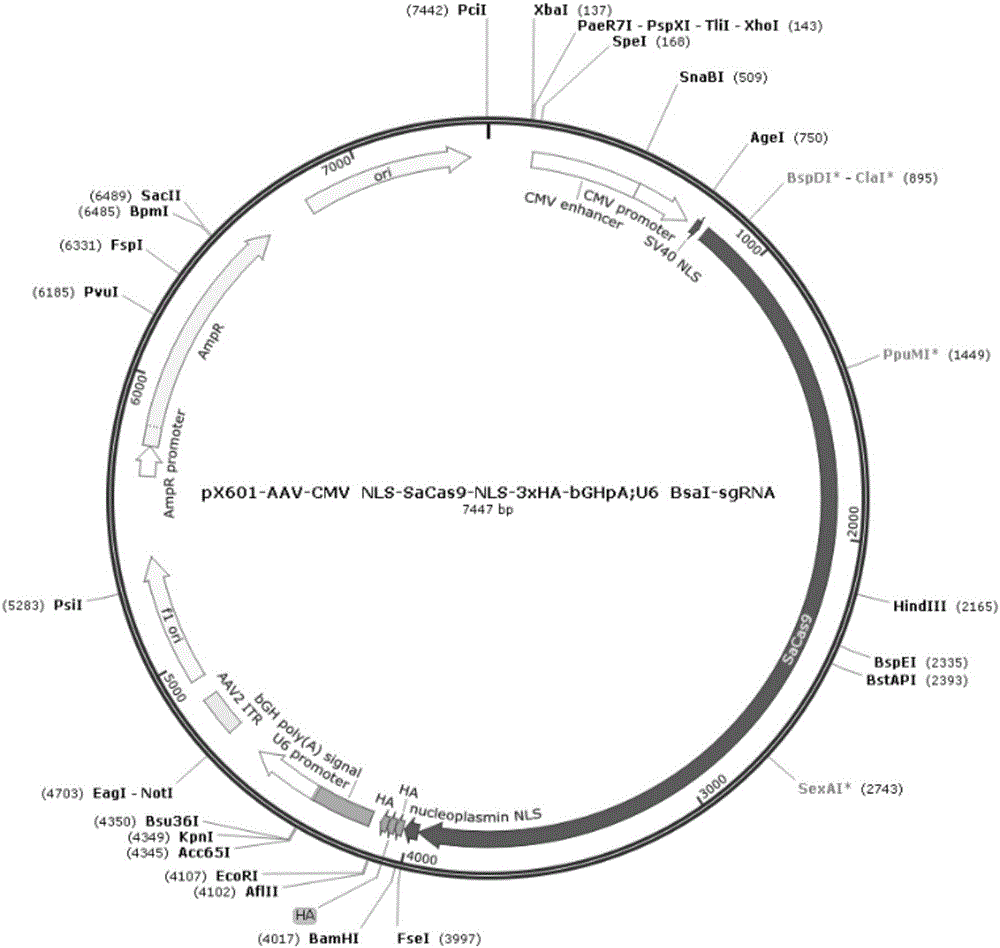
[0078] 1) The px601-AAV-CMV plasmid (map such as figure 1 shown, hereinafter abbreviated as px601) for enzyme digestion to obtain the linearized px601 plasmid; the enzyme digestion system is as follows:
[0079] 1μg px601 plasmid;
[0080] 2μl 10×FastDigest® buffer;
[0081] 1 μl FastDigest® BsaI (Thermo Scientific);
[0082] Add water to 20 μl, incubate at 37°C for 1 hour, and then cut the gel for recovery.
[0083] 2) Connect
[0084] The double-stranded DNA oligonucleotides (TCR-DNA Oligos-1-4) obtained in Example 2 were respectively connected with the linearized px601, and the connection system was as follows:
[0085] 2.5μl px601 plasmid;
[0086] 2.5 μl of annealed double-stranded sgRNA;
[0087] 5 μl Solution I (Takara);
[0088] Incubate for 1 hour at 16°C.
[0089] 3) Transformation: The above ligation product was transformed into E. coli DH5α competent cells, incubated at 37°C for 16-18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com