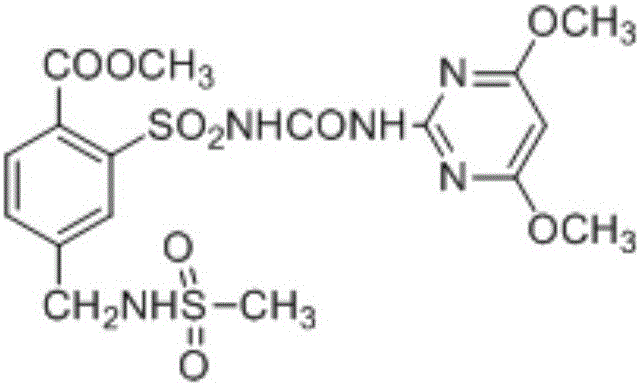
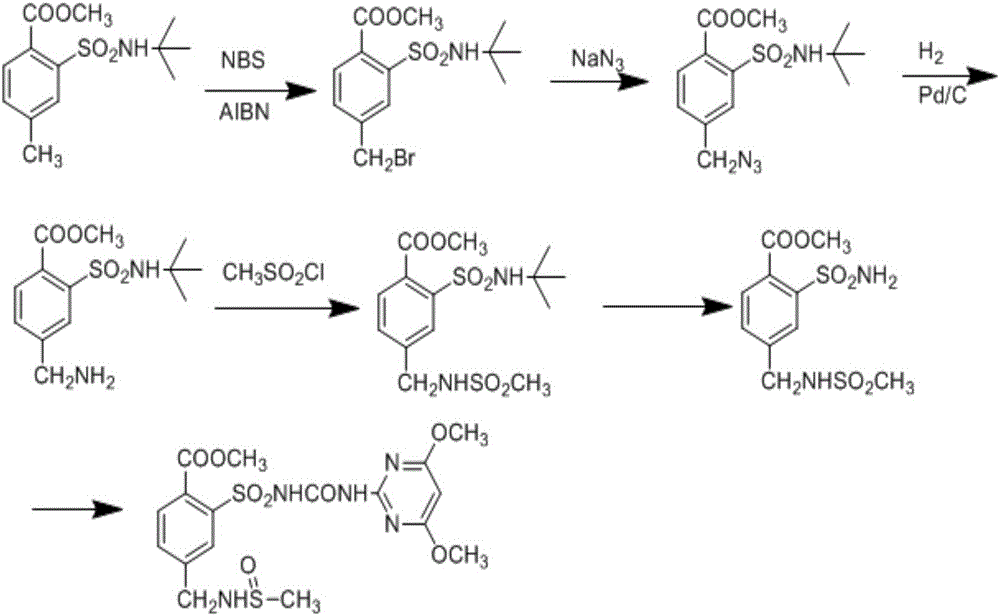
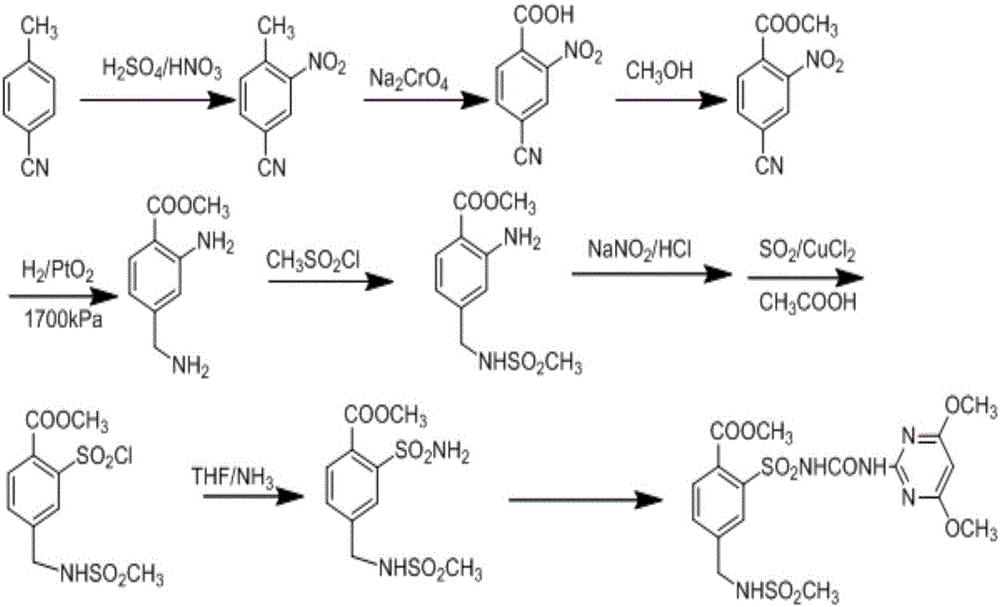
Preparation method of mesosulfuron
A technology for the synthesis of methylsulfuron-methyl, which is applied in the field of pesticide chemistry, can solve the problems of cumbersome synthesis steps, cumbersome and complicated operations, and difficult availability of raw materials, and achieve the effects of cheap and easy-to-obtain raw materials, simple operation, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A kind of synthetic method of methylsulfuron methyl, its steps are as follows:
[0052] 1) Preparation of 4-chloro-2-aminobenzoic acid methyl ester (2)
[0053] Add 17.1g of 4-chloro-2-aminobenzoic acid (0.1mol) and 50mL of methanol into the reaction kettle, stir to dissolve, cool to -5~10°C, slowly add 5mL of thionyl chloride dropwise, after the dropwise addition, remove Ice bath, slowly raised to room temperature, stirred for 0.5h, heated the reaction solution to reflux, reacted for 4-5h, distilled off and added 30mL of methanol, and applied mechanically next time, added the residue into ice water, added sodium bicarbonate to adjust it to neutral properties, filter, and dissolve the filter cake with 100mL of dichloromethane, filter to obtain 2g of unreacted 4-chloro-2-aminobenzoic acid, which can be directly applied to the next esterification reaction, and use 5g of anhydrous sodium sulfate for the dichloromethane layer Dry, filter, and distill off the solvent to obt...
Embodiment 2
[0072] A kind of synthetic method of methylsulfuron-methyl, the steps are as follows:
[0073] 1) Preparation of 4-chloro-2-aminobenzoic acid methyl ester (2)
[0074] Add 17.1g of 4-chloro-2-aminobenzoic acid (0.1mol) and 100mL of methanol into the reaction kettle, stir to dissolve, cool to -5~0°C, slowly add 10mL of thionyl chloride dropwise, after the dropwise addition, remove Ice bath, slowly raised to room temperature, stirred for 0.5h, heated the reaction solution to reflux, reacted for 4h, distilled off and added 70mL of methanol, and applied mechanically next time, added the residue into ice water, added sodium bicarbonate to adjust it to neutral, Filter, dissolve the filter cake with 100 mL of dichloromethane, filter to obtain 1.8 g of unreacted 4-chloro-2-aminobenzoic acid, which can be directly applied to the next esterification reaction, and dry the dichloromethane layer with 5 g of anhydrous sodium sulfate , filtered, and the solvent was distilled off to obtain 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com