PH response/membrane adhesive amphiphilic block copolymer as well as preparation method and application thereof
A technology of amphiphilic block and copolymer, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, emulsion delivery, etc. It can solve problems such as complex preparation process, negative charge, and functional limitations of membrane adhesion. , to prolong the residence time and promote the effect of drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
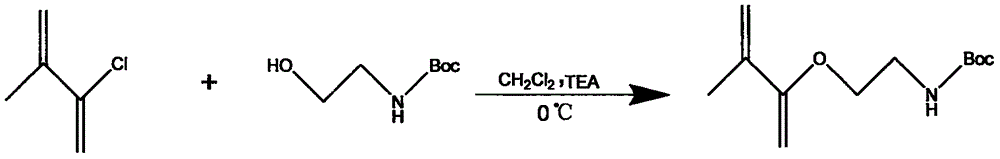
[0083] (1) Synthesis of membrane-adhesive monomer tert-butoxycarbonyl (Boc)-protected aminoethyl methacrylate (Boc-AEMA)
[0084] Take a 100ml dry round-bottomed flask and place it in an ice-salt bath, add 10mL of absolute ethanolamine and 40mL of dichloromethane, stir magnetically for 15min, dissolve 30g of di-tert-butyl dicarbonate (O) in 50ml of dichloromethane and slowly add it dropwise to Burn in the round bottom bottle, continue to stir in the ice-salt bath for 20 minutes after the dropwise addition, and then transfer to room temperature to react for 12 hours. After the reaction, wash the reaction solution with 10% solution for 3 times, and then wash with deionized water for 3 times , take the organic phase and dry it with an appropriate amount of anhydrous for 2 hours. After drying, filter under reduced pressure and rotary evaporation to obtain a colorless transparent viscous liquid, and dry it in vacuum at 40°C for 24 hours to obtain Boc-protected ethanolamine.
[0085...
Embodiment 2
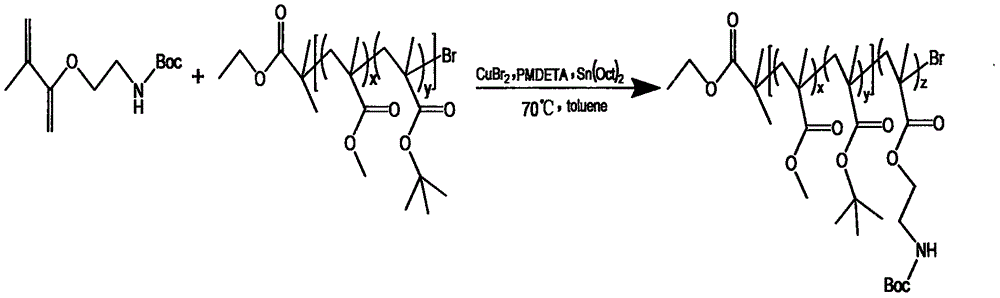
[0093] (1) The synthesis of the membrane-adhesive monomer tert-butoxycarbonyl (Boc)-protected aminoethyl methacrylate (Boc-AEMA) is the same as the step (1) in Example 1.
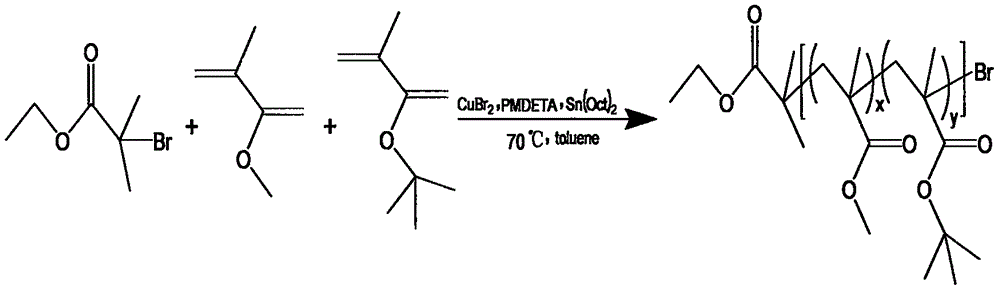
[0094] (2) Synthesis of macromolecular initiator P(MMA-co-tBMA)-Br (A:B=30:45)
[0095] Take a 50ml dry eggplant-shaped bottle, weigh (9mg, 0.04mmol) and put it in it, seal it with a rubber stopper, evacuate and blow argon for 3 times, and inject toluene (12mL), MMA and (3.180mL, 30mmol), tBMA (7.35mL, 45mmol) and the ligand PMDETA (0.130mL) were added to the bottle, stirred magnetically for 30min to form a catalyst complex, and the reducing agent (0.195mL, 0.6mmol) was dissolved in 3ml of toluene and injected into In the reaction flask, the stirring was continued for 30 min, and the freezing-pumping-ventilating-heating cycle was performed 3 times with liquid nitrogen, the initiator EBriB (0.147ml) was injected, and the reaction was carried out in a 75°C oil bath for 2h. After the reaction is complete, coo...
Embodiment 3
[0101] Determination of critical micelle concentration of a pH-responsive / membrane-adhesive amphiphilic block copolymer by pyrene fluorescent probe method.
[0102] (1) Prepare a certain concentration of pyrene solution: accurately weigh 2.43mg of pyrene and dissolve it in 10ml of acetone, pipette it into a 100ml volumetric flask, and dilute it with acetone to make a concentration of 12×10 -5 The pyrene solution of M is ready for use. Take 5ml 12×10 -5 The pyrene solution of M was diluted with acetone to 6×10 -5 M.
[0103] (2) Preparation of sample solution: Weigh 10mg of amphiphilic block copolymer (product of Example 1) P(MMA-co-MAA)-b-P(AEMA) and dissolve it in 3ml of acetone, and accurately add 10ml of deionized under stirring water, stirred overnight to completely volatilize the acetone to obtain a 1mg / ml polymer mother liquor, dilute the polymer mother liquor into a series of 0.0001~1mg / ml solutions, take 10 10ml clean volumetric flasks, add 0.1ml 6×10 -5 M pyrene s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com